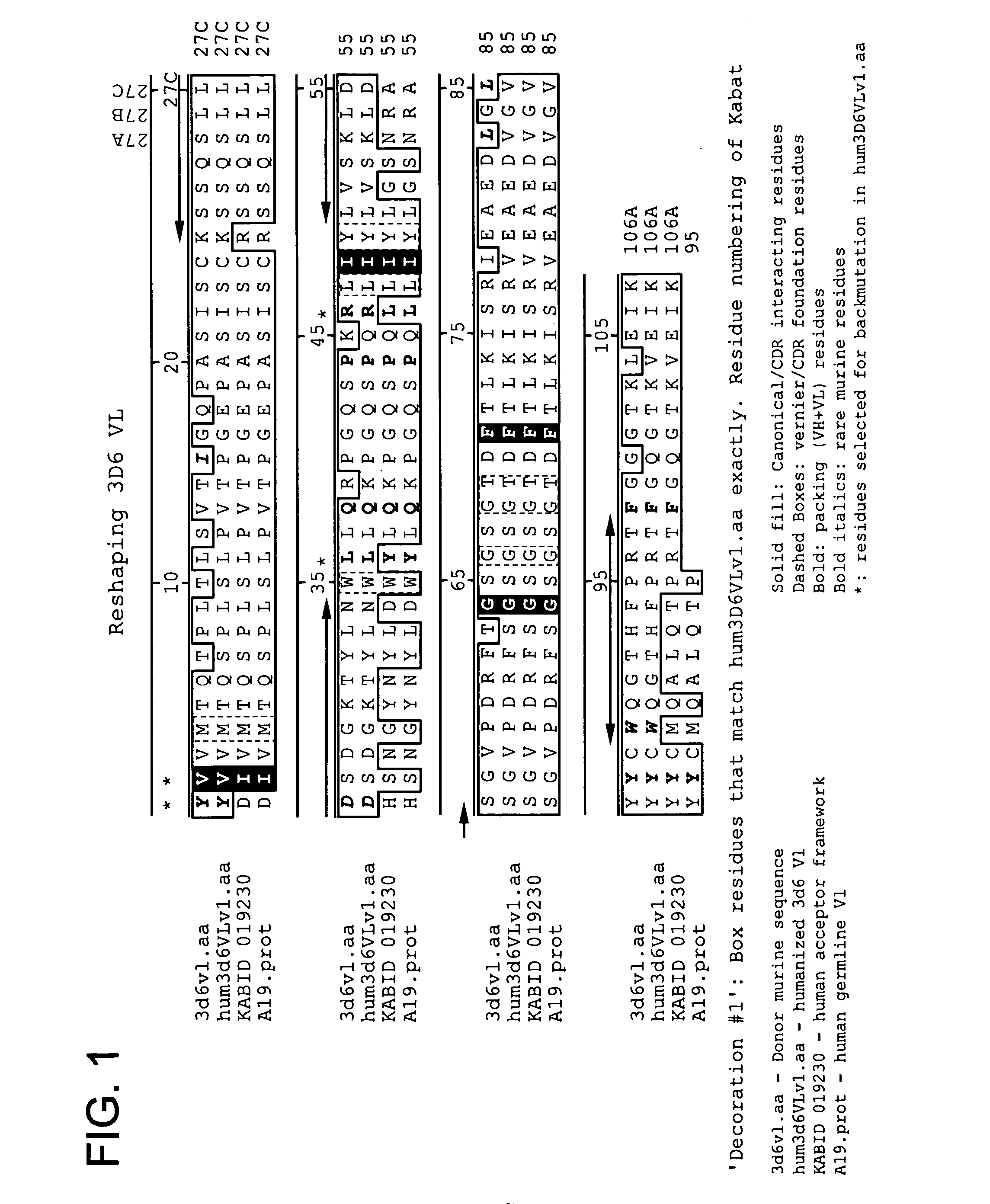
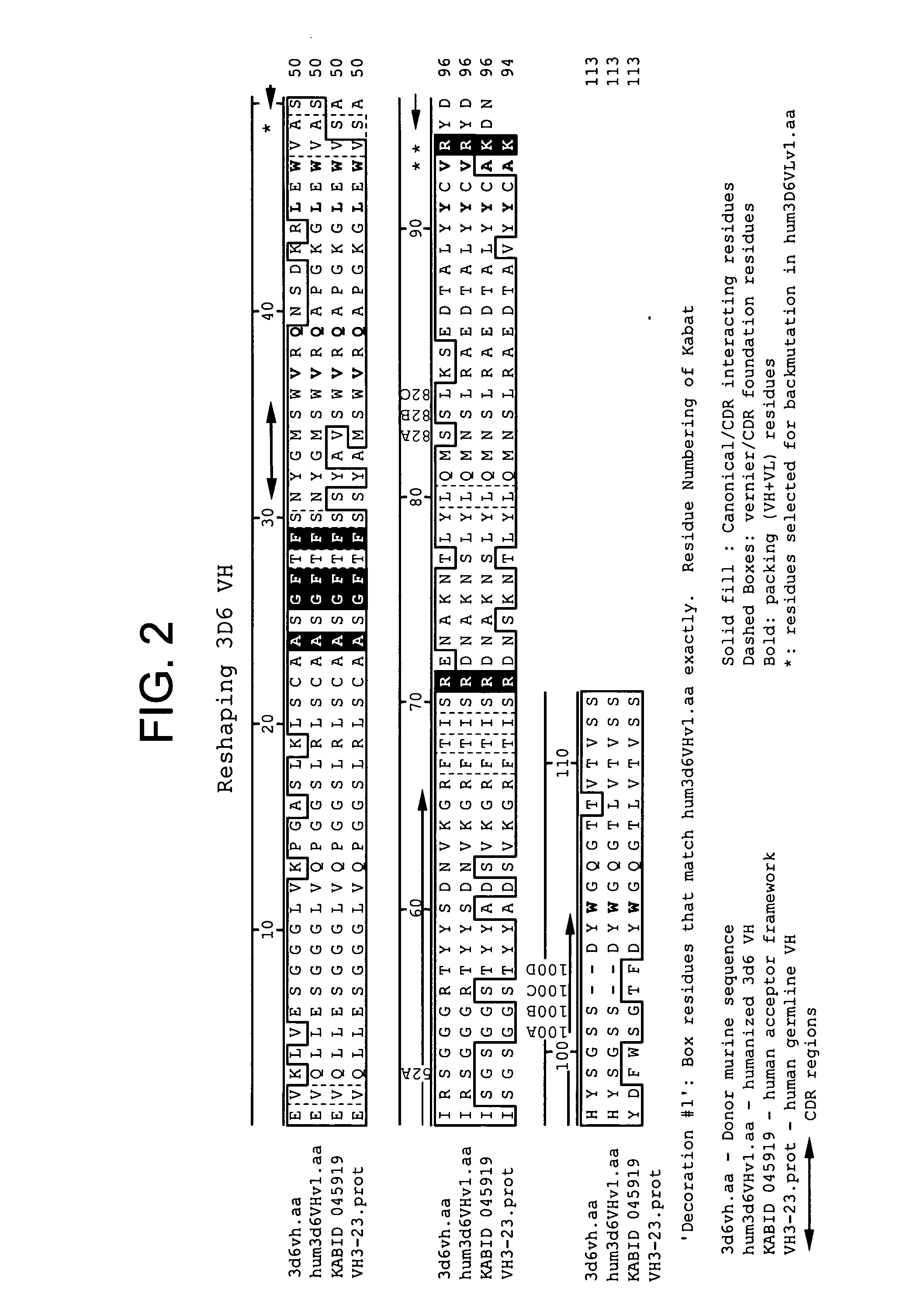
Humanized antibodies that recognize beta amyloid peptide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Therapeutic Efficacy of Anti-Aβ Antibodies
mAb 2H3, mAb 10D5, mAb 266, mAb 21F12 and pAb Aβ1-42
[0255] This example tests the capacity of various monoclonal and polyclonal antibodies to Aβ to inhibit accumulation of Aβ in the brain of heterozygotic transgenic mice.
[0256] A. Study Design
[0257] Sixty male and female, heterozygous PDAPP transgenic mice, 8.5 to 10.5 months of age were obtained from Charles River Laboratory. The mice were sorted into six groups to be treated with various antibodies directed to Aβ. Animals were distributed to match the gender, age, parentage and source of the animals within the groups as closely as possible. Table 2 depicts the Experimental design.
TABLE 2Experimental DesignTreatmentTreatmentAntibodyAntibodyGroupNaAntibodySpecificityIsotype19noneNAbNA(PBS alone)210PolyclonalAβ1-42mixed30mAbd 2H3Aβ1-12IgG148mAb 10D5Aβ3-7IgG156mAb 266Aβ13-28IgG168mAb 21F12Aβ33-42IgG2a
aNumber of mice in group at termination of the experiment. All groups started with 10 an...
example ii
Therapeutic Efficacy of Anti-Aβ Antibodies
mAb 2H3, mAb 10D5, mAb 266, mAb 21F12, mAb 3D6, mAb 16C11 and pAb Aβ1-42
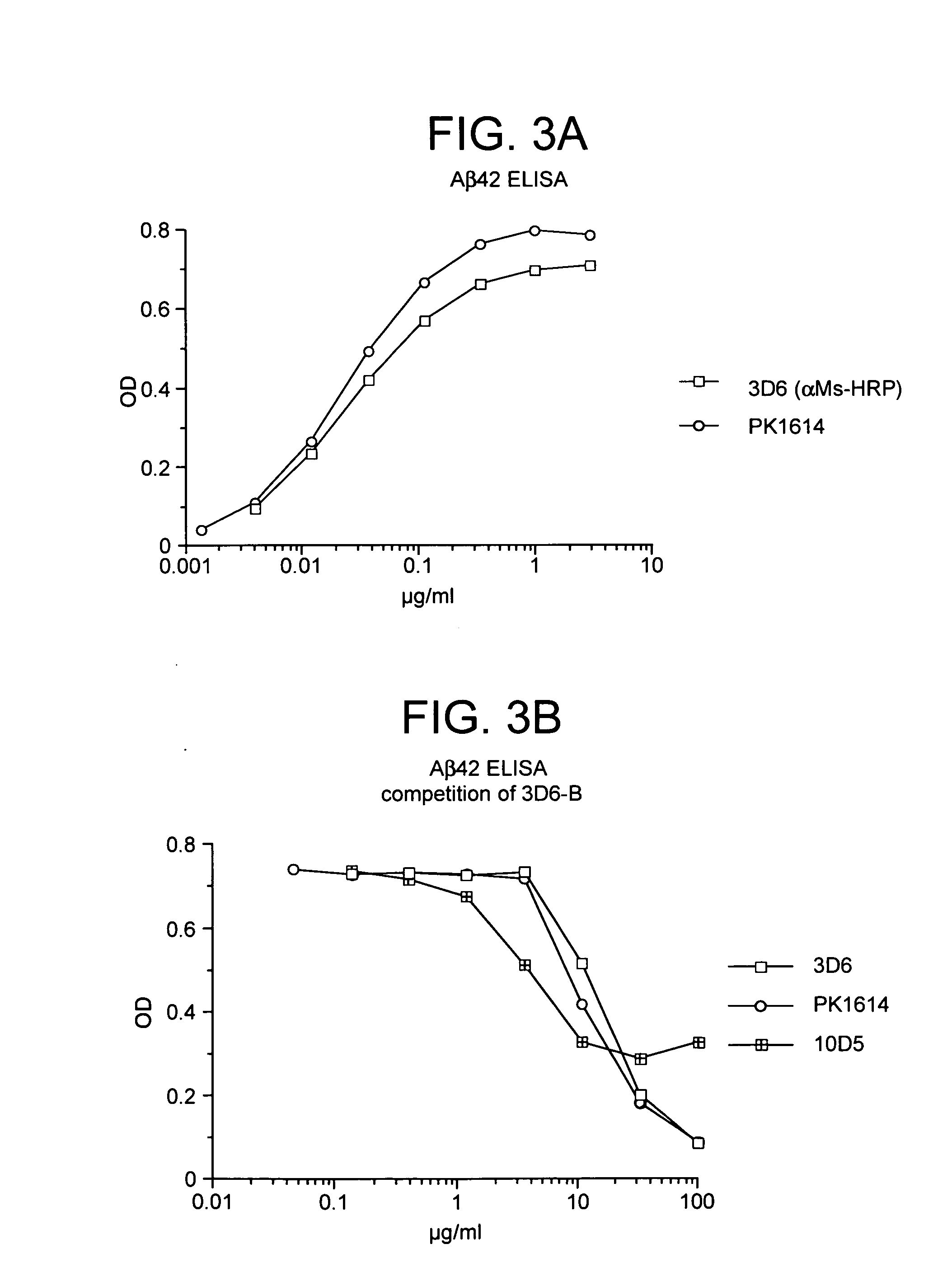
[0279] In a second study, treatment with 10D5 was repeated and two additional anti-Aβ antibodies were tested, monoclonals 3D6 (Aβ1-5) and 16C11 (Aβ33-42). Control groups received either PBS or an irrelevant isotype-matched antibody (TM2a). The mice were older (11.5-12 month old heterozygotes) than in the previous study, otherwise the experimental design was the same. Once again, after six months of treatment, 10D5 reduced plaque burden by greater than 80% relative to either the PBS or isotype-matched antibody controls (p=0.003). One of the other antibodies against Aβ, 3D6, was equally effective, producing an 86% reduction (p=0.003). In contrast, the third antibody against the peptide, 16C11, failed to have any effect on plaque burden. Similar findings were obtained with Aβ42 ELISA measurements.
[0280] These results demonstrate that an antibody response against Aβ pepti...
example iii
Monitoring of Antibody Binding in the CNS
[0281] This Example demonstrates that when held at modest serum concentrations (25-70 μg / ml), the antibodies gained access to the CNS at levels sufficient to decorate β-amyloid plaques.
[0282] To determine whether antibodies against Aβ could be acting directly within the CNS, brains taken from saline-perfused mice at the end of the Example II, were examined for the presence of the peripherally-administered antibodies. Unfixed cryostat brain sections were exposed to a fluorescent reagent against mouse immunoglobulin (goat anti-mouse IgG-Cy3). Plaques within brains of the 10D5 and 3D6 groups were strongly decorated with antibody, while there was no staining in the 16C11 group. To reveal the full extent of plaque deposition, serial sections of each brain were first immunoreacted with an anti-Aβ antibody, and then with the secondary reagent. 10D5 and 3D6, following peripheral administration, gained access to most plaques within the CNS. The plaq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com