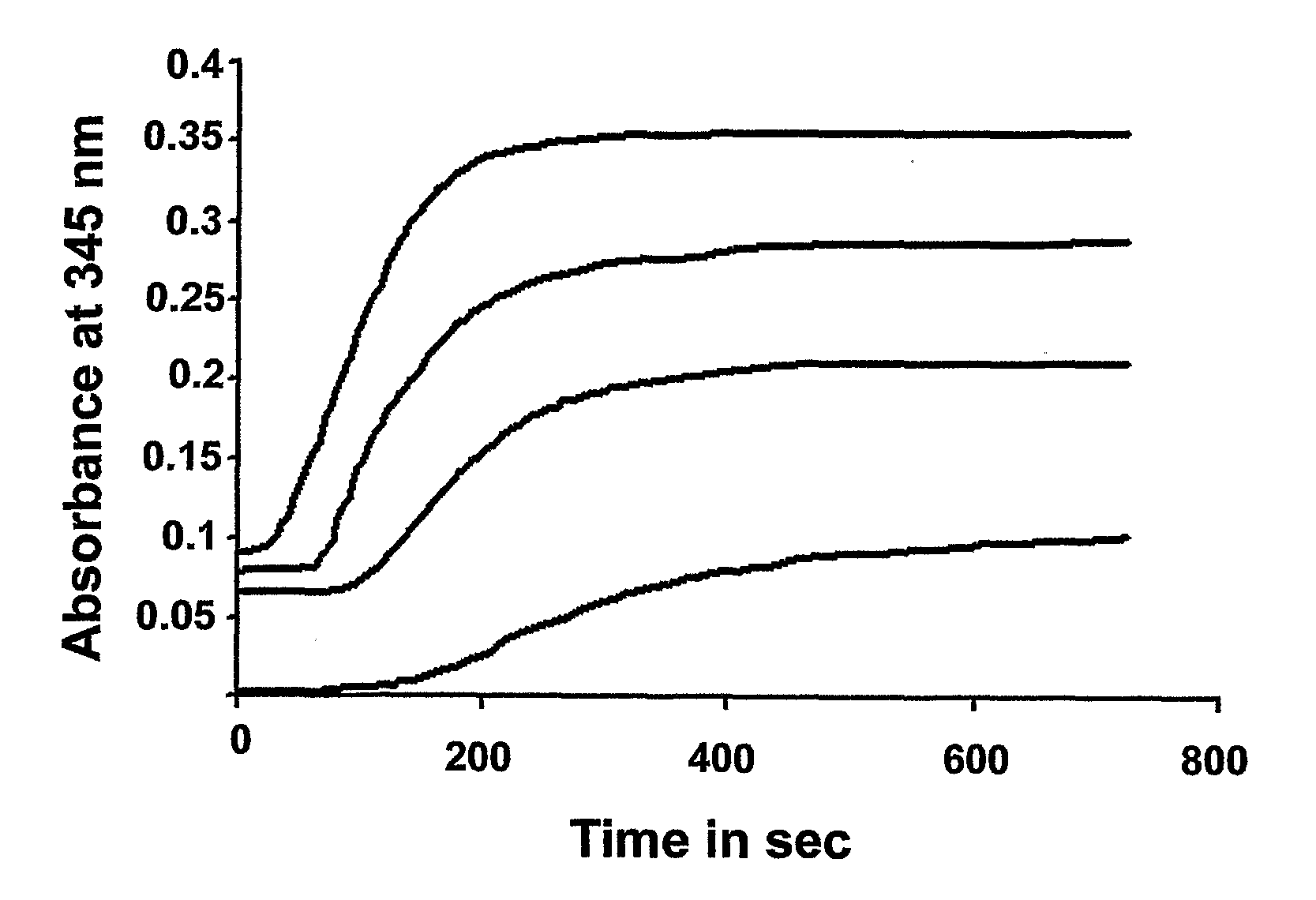
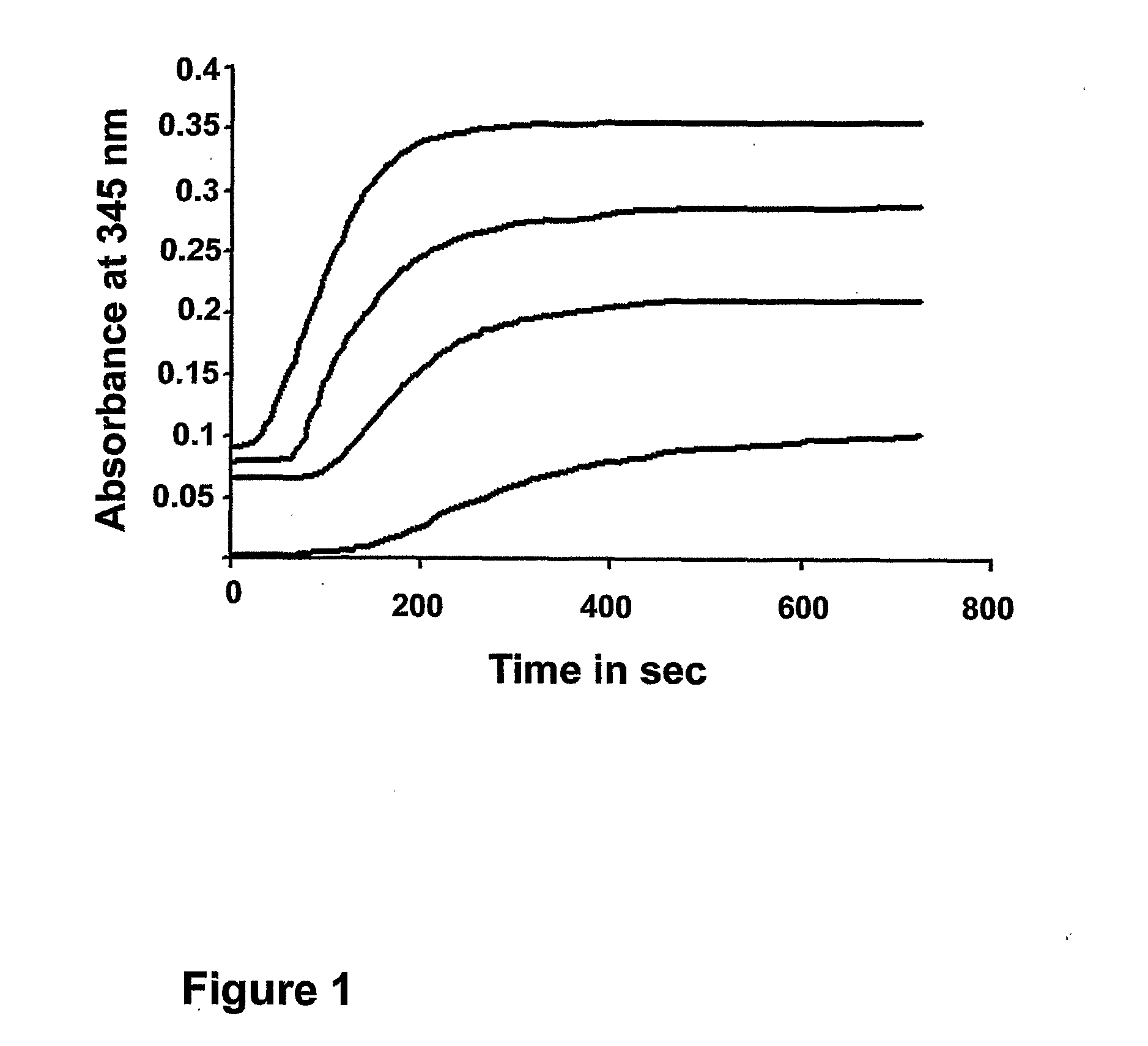
Dat1
a technology of diaminoketothiazole and dat1, which is applied in the field of diaminoketothiazole, can solve the problems of high cost and achieve the effect of improving the safety and efficacy of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Conversion of aminomethylpolystyrene (AMPS) to N—(N-arylthiocarbamoyl)-N′-guanidinomethyl polystyrene 2 (AGMPS)
General Procedure
[0029]Aminomethylpolystyrene resin beads (2 g, 2.13 meq. NH2 / g resin) was swelled in acetonitrile (5 ml). To the swelled resin, a solution of 1-[(N-arylthiocarbamoyl)amidino]-3,5-dimethylpyrazole 1 (2 molar equivalents) in acetonitrile (10 mL) was added. The mixture was then refluxed for 12-15 h. The resin beads were then removed by filtration, washed repeatedly with warm and then cold acetonitrile (3×10 ml), then with petroleum ether (60-80° b.p) (2×10 ml) and then dried in vacuum. The S capacity of the resin was then estimated by digestion and gravimetry by standard procedures. This was found to be in the range 0.98-1.32 meg / g resin.
2. Synthesis of 5-acyl-4-amino-2-arylaminothiazoles 5
General Procedure:
[0030]The above arylthiocarbamoyl resin (AGMPS) was swelled in N,N-dimethyl formamide (DMF) (5 ml). To this, the respective α-bromoketone (molar equival...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| Dissociation constant | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com