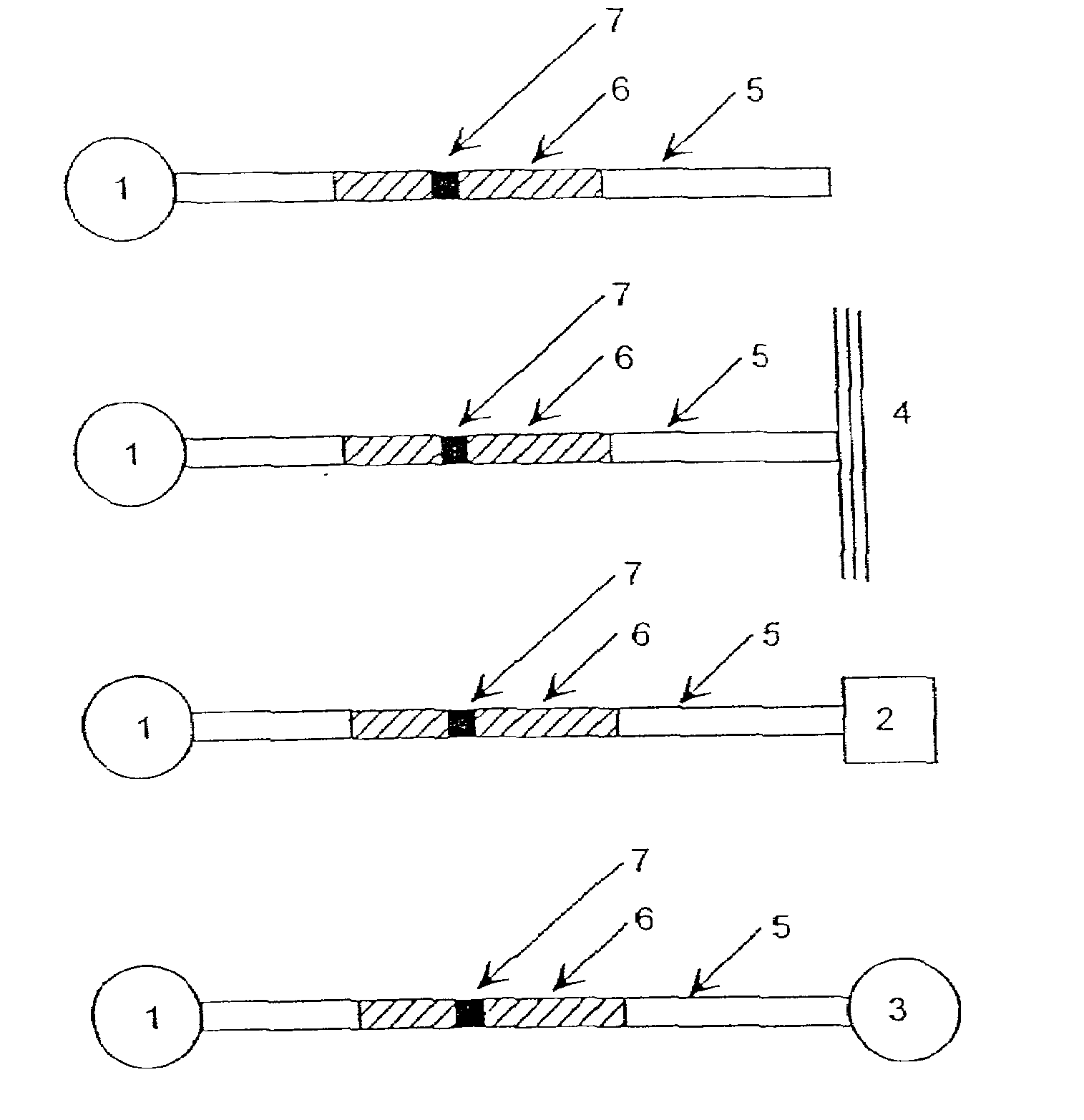
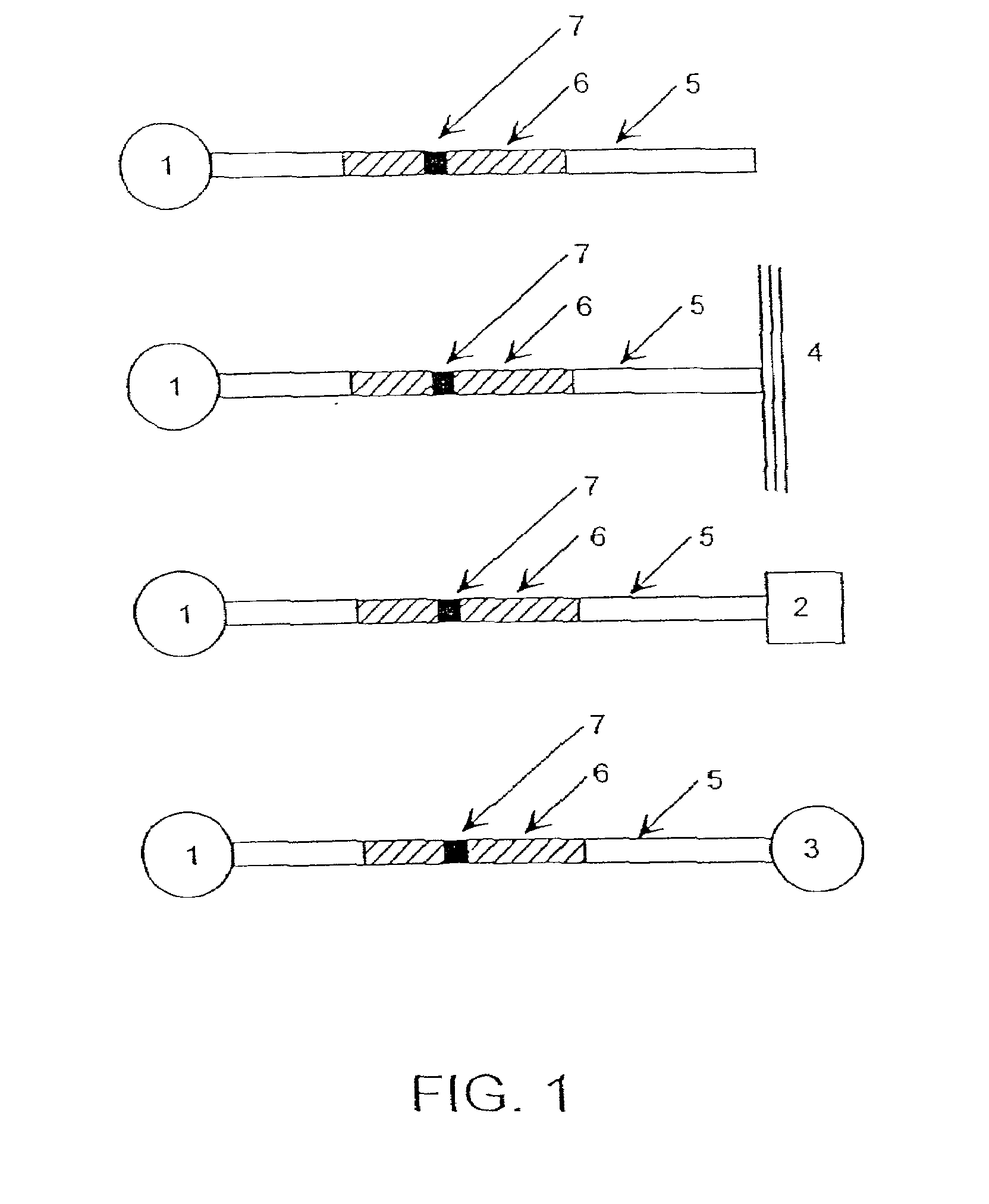
Optical probes and assays
a type modification and probe technology, applied in the field of chemistry and biology, can solve the problems of preventing or hampering the ability to rapidly screen for drugs using miniaturized automated formats, placing considerable constraints on the assays that can be successfully employed, and few existing methods of measuring such activities that are homogenous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Measurement of Tyrosine Kinase Activity Using Optical Probes
[0162]Peptides were prepared by traditional solid-phase synthesis see, Merrifield, J. Amer. Chem. Soc., 85:2149-2154 (1963); Fields, G. B., et al., Principles and practice of solid-phase peptide synthesis, pages 77-183 in Synthetic Peptides: A Users Guide, Grant, G. R., ed., W. H. Freeman and Co. New York, (1992), in conjunction with the “tea-bag” methodology using Boc / benzyl based chemistry. See, Houghten et al., Proc. Natl. Acad. Sci. USA 82:513-5135 (1985). Peptides were assembled on methylbenzhydrylamine resin (MBHA resin) using traditional Boc / Benzyl based chemistry. A minor modification to the protocol (in the case of the abl-specific substrate (AEAIYAAPL, SEQ. I.D. No. 4) was the use of a base sensitive protecting group (Fmoc) for the side chain of the C-terminal lysine residue. Bags, made of a polypropylene mesh material were filled with MBHA resin. The bags (“tea-bags”) were placed in a Nalgene™ bottle with dichlor...
example 2
Validation of Optical Probes for Screening for Protein Tyrosine Kinase Inhibitors
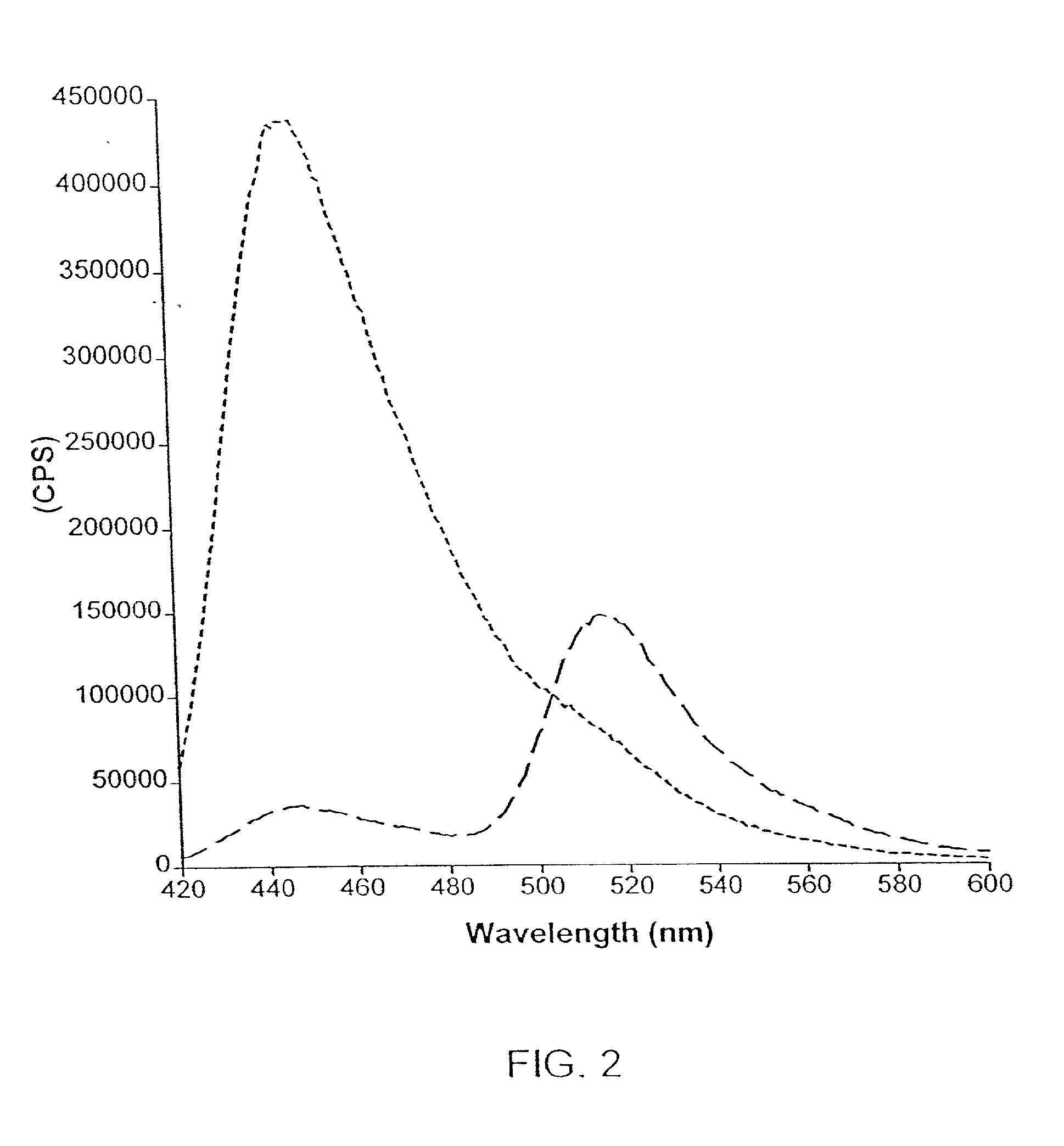
[0177]To validate the invention in a high throughput screening format, optical probe-based assays were carried out in a 96-well plate reader. The results demonstrated highly reproducible and accurate results with the present invention. As shown in Table 9, the calculation of emission ratios significantly reduces the standard deviation and C.V. values compared to intensity measurements at either 460 or 530 nm. The reduction of errors is an important consideration in the design and analysis of screening systems, and particularly automated high throughput and ultra-high through screening systems.
TABLE 9EmissionEmission460 nm530 nmRatioMean129019220.67Standard3.74.80.01DeviationC.V.2.9%2.5%1.5%
[0178]Analysis of the kinetics of phosphorylation of the optical probe revealed values for the apparent Km for the substrate of 40 μM, and an apparent Km for ATP of 8 μM. The turnover of the optical probe by v-Abl was...
example 3
Measurement of Other Tyrosine Kinase Activities Using Optical Probes
Measurement of Src Kinase Activity
[0181]To measure Src kinase activity, two optical probes Src-1 (GEEEIYGEIEK, SEQ. ID. NO: 3) and Src-2 (GEEEIYGVIEK, SEQ. ID. NO: 29) were developed. In the case of the Src-1 kinase substrate, and as shown in Table 4, a second aromatic amino acid was changed to isoleucine in the optical probe. In the second substrate, Src-2, the negatively charged amino acid (Glu=E) in the P′2 position with respect to the protease site, was changed to valine (Val=V) to enable more efficient cleavage of the non-phosphorylated optical probe by chymotrypsin. Src kinase (Upstate Biotechnology) reaction conditions were the same as described in Example 1, except 25 mM glycerol phosphate and 1 mM DTT were also added. Incubation of the optical probe with chymotrypsin (100 nM) and measurement of fluorescence emission ratios were as described in Example 1. The apparent Kms for the two substrates, (determined ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| fluorescent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com