Agonist anti-trkc antibodies and methods using same
a technology of agonist and antitrkc antibody, which is applied in the direction of peptides, drug compositions, and infusion cells, can solve the problems of neurotrophin therapy, neurotrophins, neurotrophins, and neurotrophins, and achieve the effects of reducing the number of nt-3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
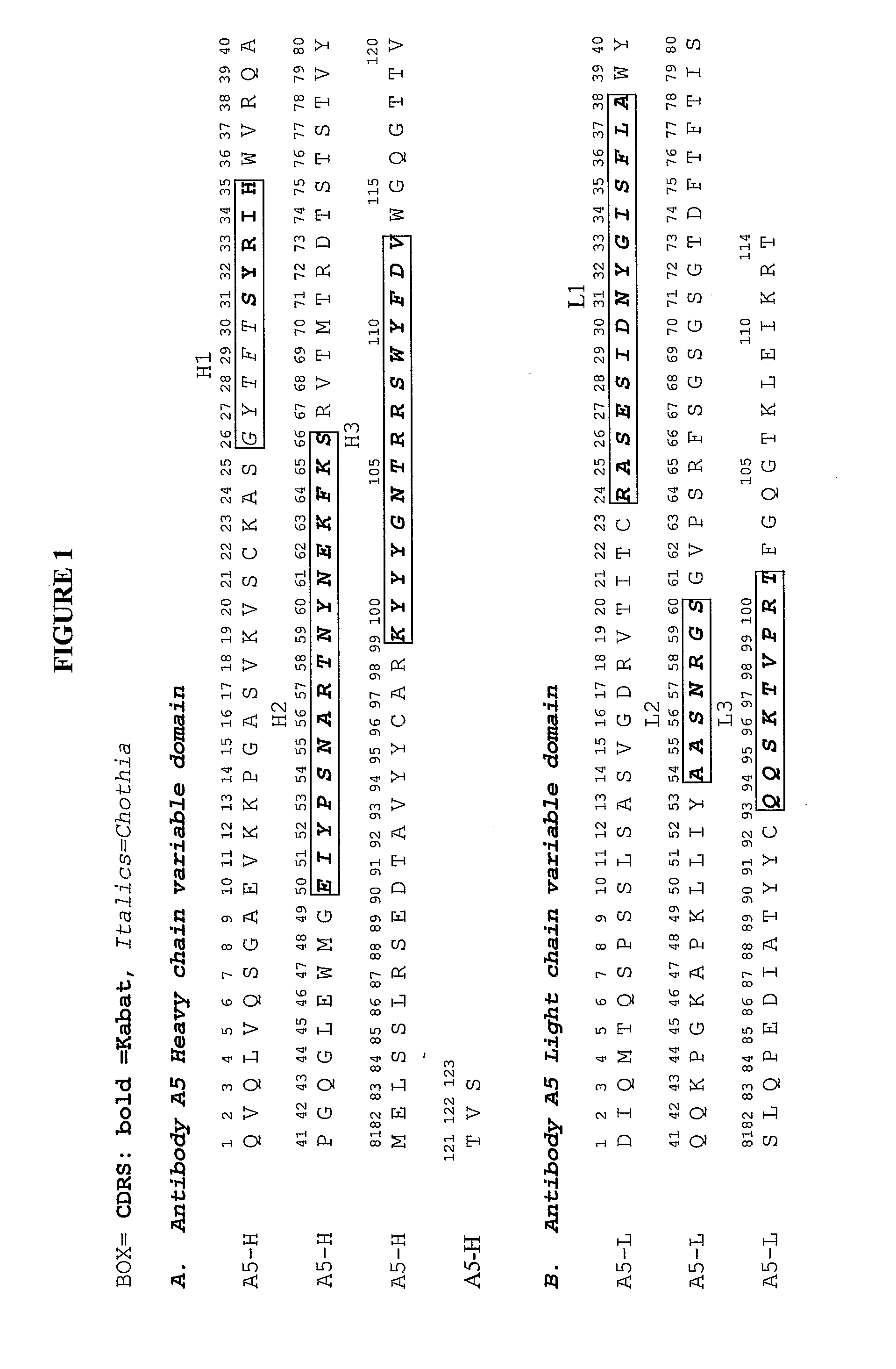
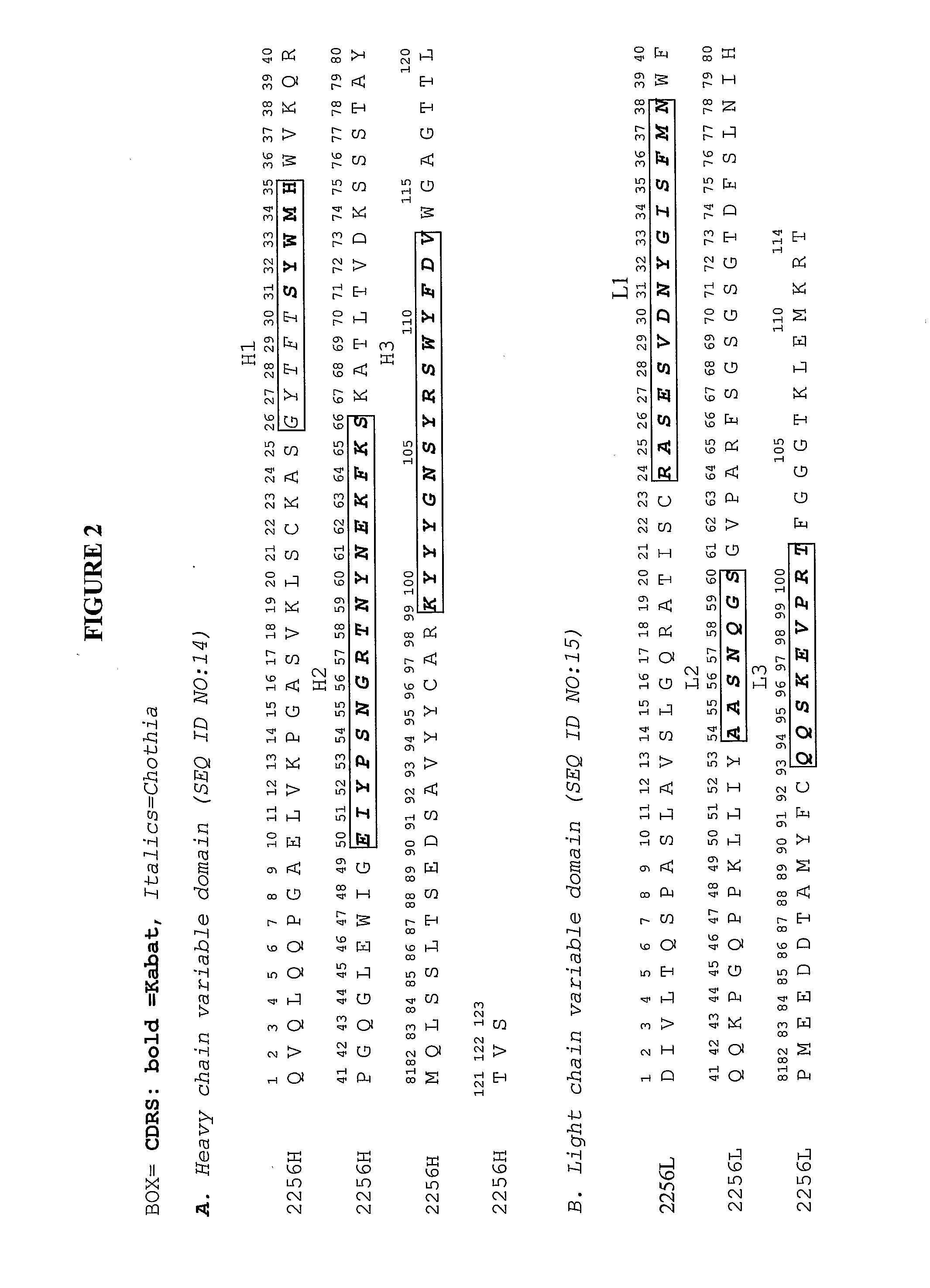
Humanization and Affinity Maturation of Mouse Monoclonal Antibody 2256
[0228]A. General Methods
[0229]The following general methods were used in this example.
[0230]Expression Vector Used in Clone Characterization
[0231]Expression of the antibodies was under control of an IPTG inducible lacZ promotor similar to that described in Barbas (2001) Phage display: a laboratory manual, Cold Spring Harbor, N.Y., Cold Spring Harbor Laboratory Press pg 2.10. Vector pComb3X), however, modifications included addition and expression of the following additional domains: the human Kappa light chain constant domain and the CH1 constant domain of IgG2a human immunoglobulin. Ig gamma-2 chain C region, protein accession number P01859; Immunoglobulin kappa light chain (homo sapiens), protein accession number CAA09181.
[0232]Small Scale Fab Preparation
[0233]Small scale expression of Fab in 96 wells plates was carried out as follows. Starting from E. coli transformed with a Fab library, colonies were picked to...
example 2
Effect of Antibody A5 on Pyridoxine-Induced Neuropathy
[0255]Treatment protocol. The experiments were carried out on adult male Sprague-Dawley rats weighing 150 to 200 g at the start of the experiment, and in compliance with approved institutional animal care and use protocols. Six to eight animals were used for each treatment group. Neuropathy was induced by the injection of pyridoxine (PDX, Sigma, St. Louis, Mo.) at 100 mg / ml in distilled water immediately before injection, and administration was performed at 400 mg / kg intraperitoneally twice a day for 8 days. A5-treated animals received A5 (5 mg / kg) by intraperitoneal injection 3 days before the start of PDX treatment, and again at 1 week after the initial dose of A5. Vector-treated animals received a single subcutaneous inoculation of 25 ul of vector QL2HNT3 (1×109 pfu / ml) in the plantar surface of both hind feet 3 days prior to the start of intoxication. Vector QL2HNT3 is a replication-incompetent, genomic herpes simplex virus-b...
example 3
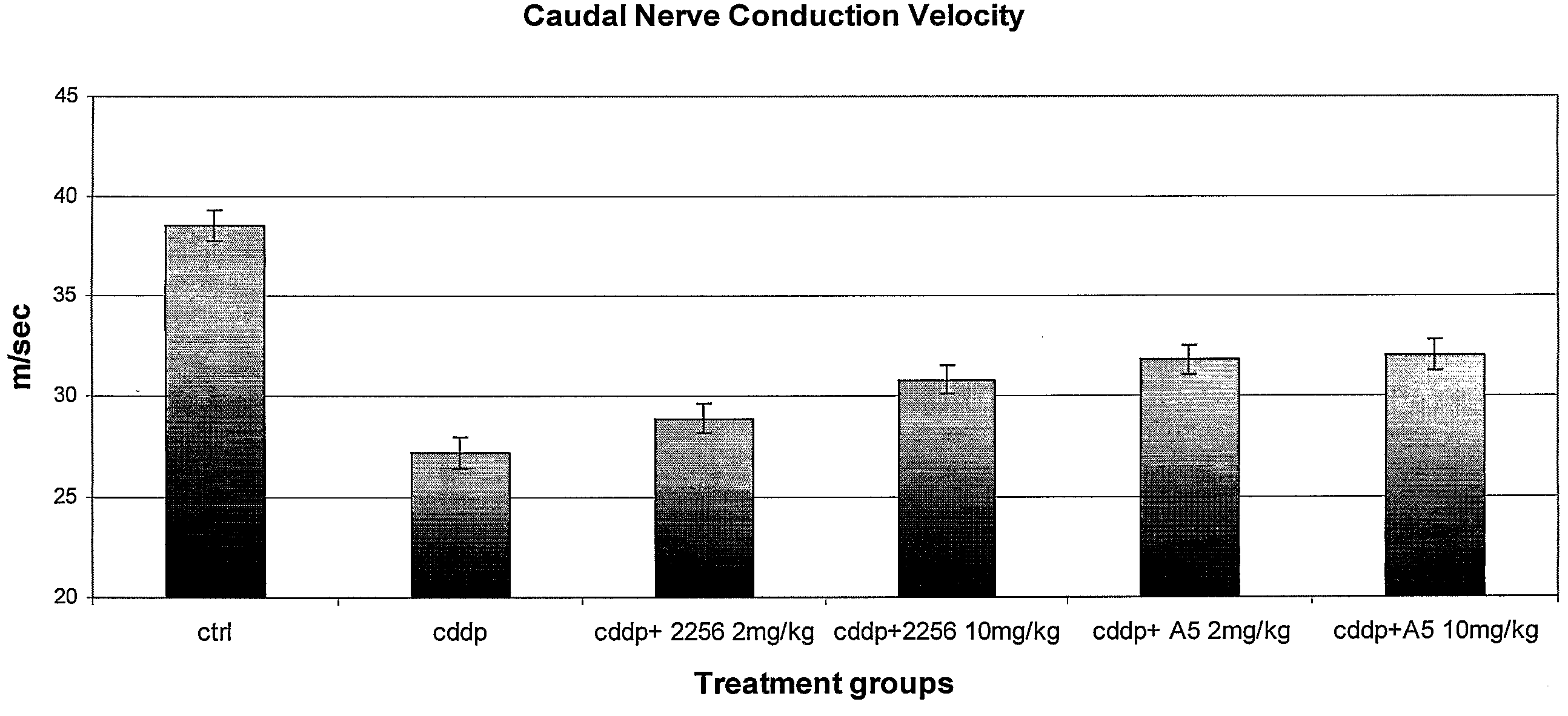
Effect of Antibody A5 on Ciplatin-Induced Neuropathy
[0260]Treatment protocol. The experiments were carried out on female Wistar rats weighing 180-200 g (Harlan, Correzzana, Italy) at the start of the experiment. Animals were divided by computer-generated random selection into the different groups and eight animals were used for each treatment group. Neuropathy was induced by intraperitoneally (ip) injection of cisplatin (CDDP) (Bristol Meyer Squibbs, at 0.5 mg / ml in sterile saline) at 2 mg / kg twice weekly for four weeks. Group 1 animals (controls) were untreated. Group 2 animals were injected with CDDP at 2 mg / kg ip twice weekly for four weeks. Group 3 animals were injected with CDDP at 2 mg / kg ip twice weekly for four weeks and subcutaneously (sc) injected with antibody 2256 at 2 mg / kg once every 7 days. Group 4 animals were injected with CDDP at 2 mg / kg ip twice weekly for four weeks and subcutaneously injected with antibody 2256 at 10 mg / kg once every 7 days. Group 5 animals were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap