Flavivirus fusion inhibitors
a technology of fusion inhibitors and flaviviruses, applied in the field of peptides, can solve the problems of frequent sexual contact, blood dialysis, accidental needle sticks, etc., and achieve the effect of reducing the load of hiv-1 and being easy to develop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029]The present invention relates to methods of inhibiting Flavivirus infection that comprises inhibiting the fusion between the virion envelope and a cell membrane, the process that delivers the viral genome into the cell cytoplasm. For purposes of clarity of disclosure, and not by way of limitation, the description of the present invention will be divided into the following subsections:
(i) peptides of the invention
(ii) utility of the invention
[0030]5.1. Peptides of the Invention
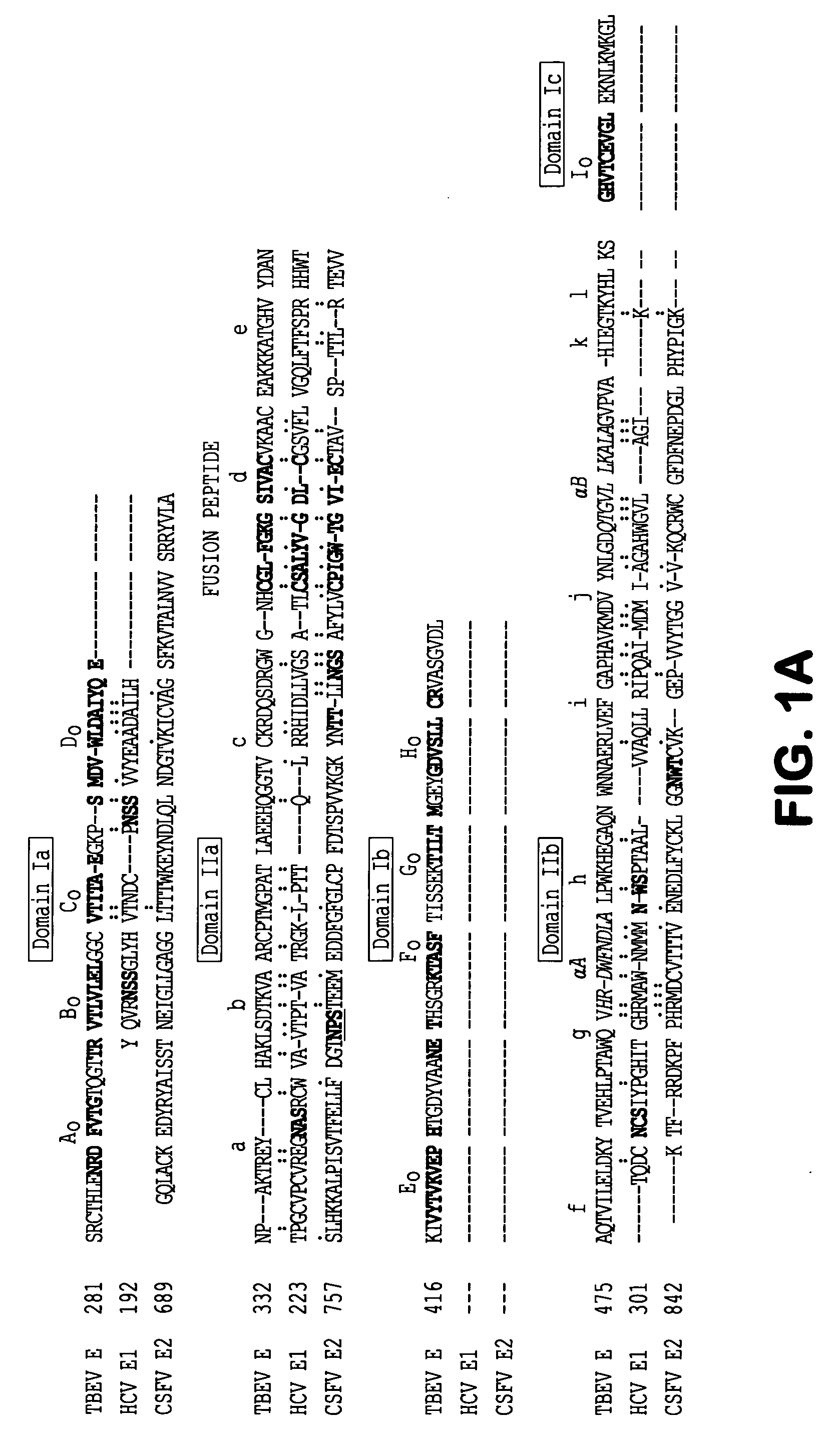
[0031]Any peptide or protein which inhibits the fusion between the Flavivirus virion envelope and a cell membrane, including those of Flaviviruses which infect human as well as nonhuman hosts, may be used according to the invention. In various embodiments of the invention, these inhibitors may include, but are not limited to peptides related to several membrane-interactive domains of Flavivirus fusion proteins.
[0032]Flavivirus inhibitory peptides are, according to the instant invention, identical or homol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com