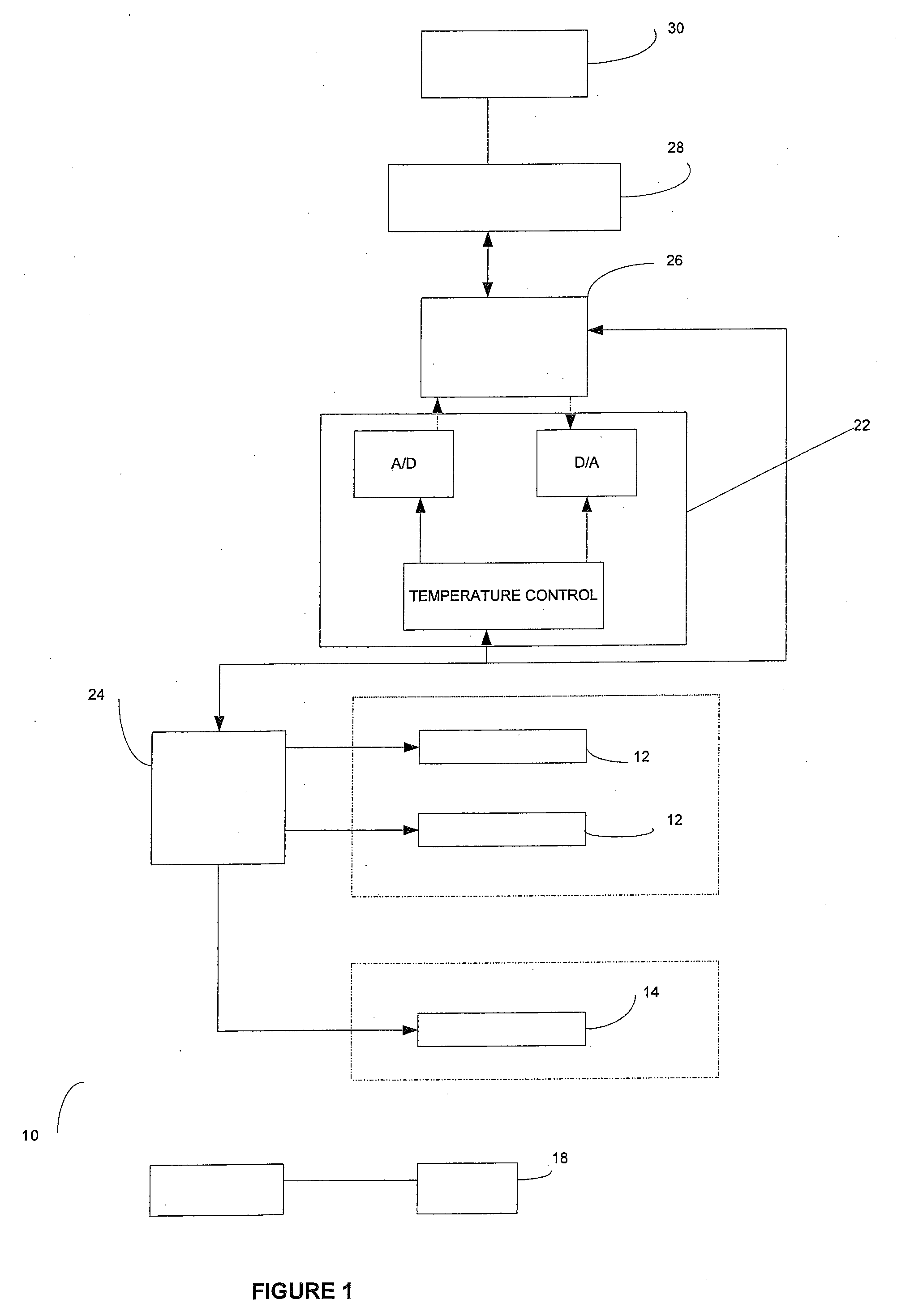Methods and Systems for Treating BPH Using Electroporation
a technology of electroporation and tissue site, applied in the field of electroporation, can solve the problems of inability to closely monitor and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
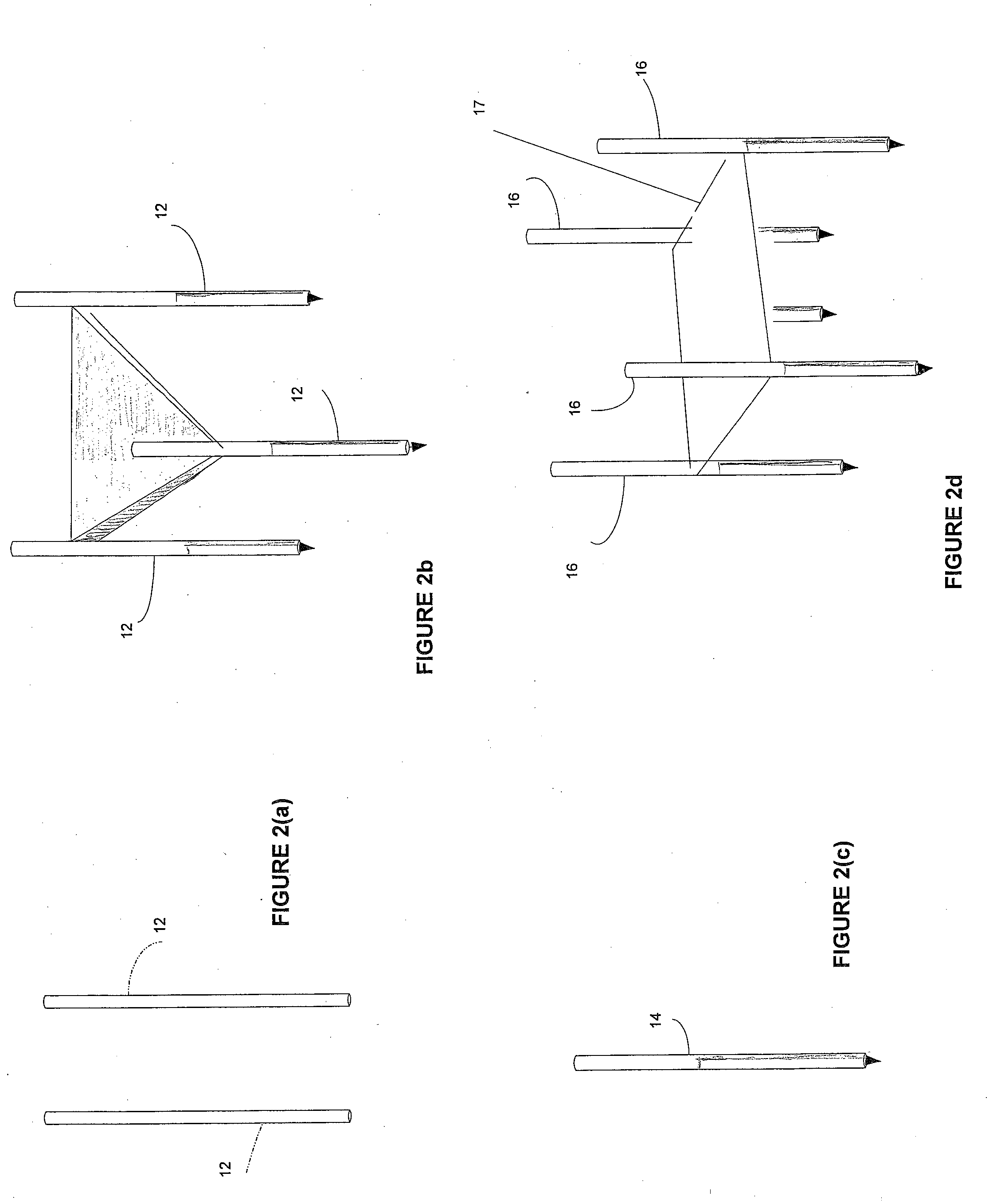
[0082]An area of the BPH tissue site is imaged. Two bi-polar electrodes 12, with sharpened distal ends, are introduced into in the BPH tissue site through the rectal wall of the patient. The area of the BPH tissue site to be ablated is positioned between the two electrodes. Imaging is used to confirm that the mono-polar electrodes are properly placed. The two mono-polar electrodes are separated by a distance of 5 mm to 10 cm at various locations of the BPH tissue site. Pulses are applied with a duration of 5 microseconds to about 62 seconds each. Monitoring is preformed using ultrasound. The BPH tissue site is monitored. In response to the monitoring, pulses are adjusted to maintain a temperature of no more than 100 degrees Celsius. A voltage gradient at the BPH tissue site in a range of from about 50 volt / cm to about 1000 volt / cm is created. A volume of the BPH tissue site of about 1 cm by 0.5 cm undergoes cell necrosis.
example 2
[0083]An area of the BPH tissue site is imaged. Two mono-polar electrodes 12, are introduced into in the BPH tissue site through the urethra of the patient. The area of the BPH tissue site to be ablated is positioned between the two mono-polar electrodes 12. Imaging is used to confirm that the electrodes are properly placed. The two mono-polar electrodes 12 are separated by a distance of 5 mm to 10 cm at various locations of the BPH tissue site. Pulses are applied with a duration of about 90 to 110 microseconds each. Monitoring is performed using a CT scan. The BPH tissue site is monitored. In response to the monitoring, pulses are adjusted to maintain a temperature of no more than 75 degrees Celsius. A voltage gradient at the BPH tissue site in a range of from about 50 volt / cm to about 5000 volt / cm is created. The BPH tissue site undergoes cell necrosis.
example 3
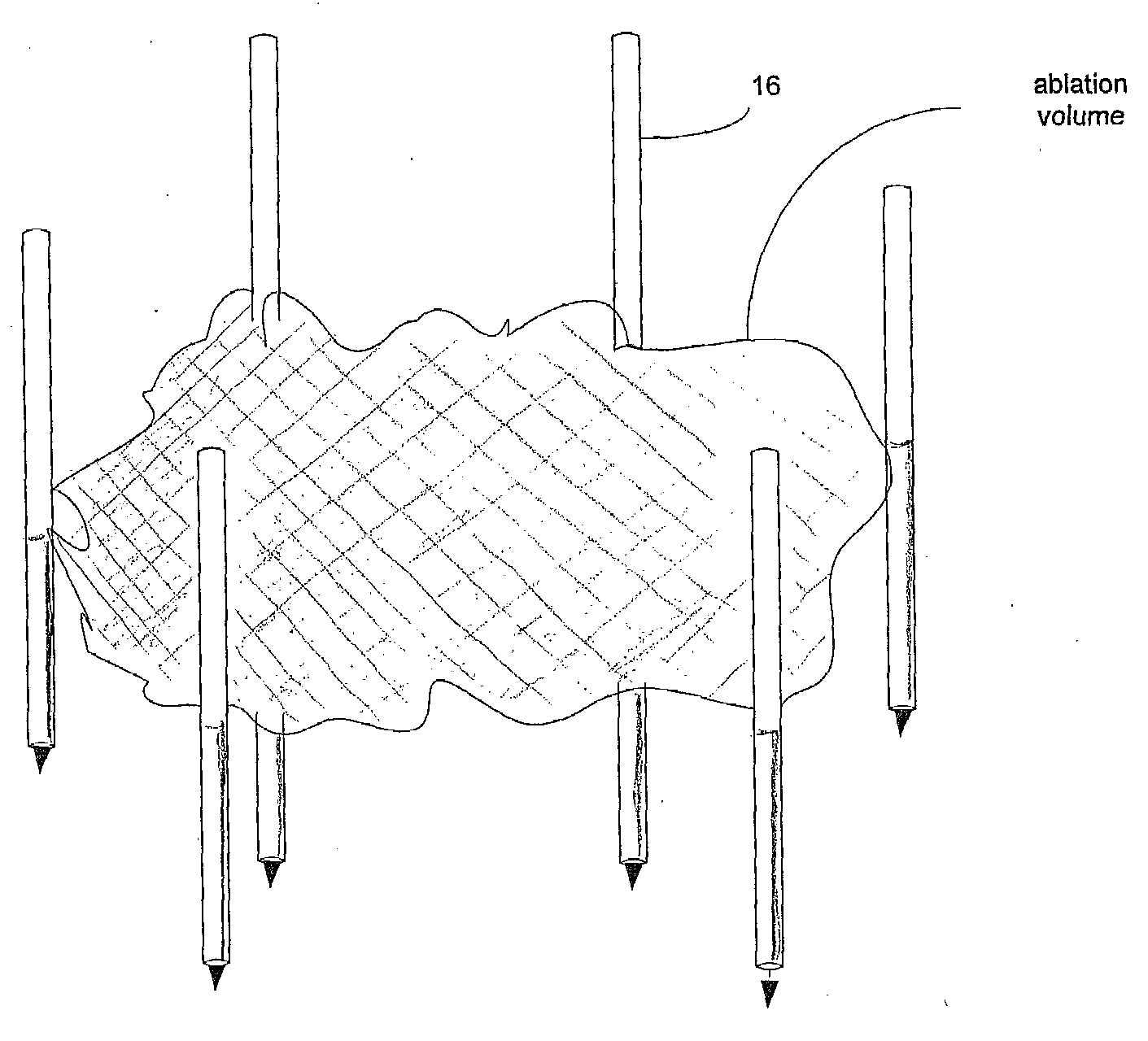
[0084]An area of the BPH tissue site is imaged. The array 16 of electrodes are introduced into in the BPH tissue site through the peritoneum of the patient. The array 16 of electrodes is positioned in a surrounding relationship to the BPH. Imaging is used to confirm that the electrodes are properly placed. Pulses are applied with a duration of about 100 microseconds each. A monitoring electrode 18 is utilized. Prior to the full electroporation pulse being delivered a test pulse is delivered that is about 10% of the proposed full electroporation pulse. The test pulse does not cause irreversible electroporation. The BPH tissue site is monitored. In response to the monitoring, pulses are adjusted to maintain a temperature of no more than 60 degrees Celsius. A voltage gradient at the BPH tissue site in a range of from about 50 volt / cm to about 8000 volt / cm is created. The BPH tissue site undergoes cell necrosis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com