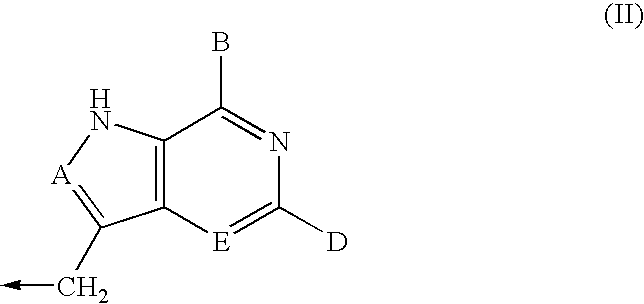
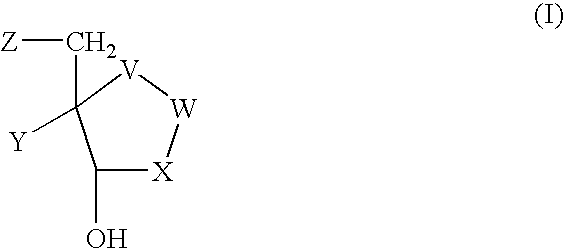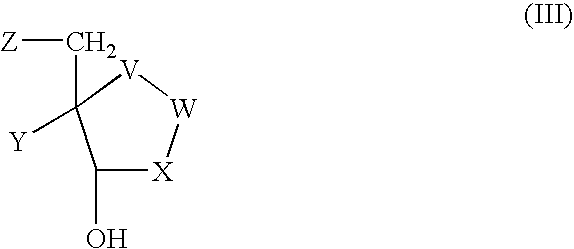Process for preparing inhibitors of nucleoside phosphorylases and nucleosidases
a nucleosidases and phosphorylase technology, applied in the field of process for preparing nucleoside analogues, can solve the problems of time and cost required for each synthesis, and achieve the effect of reducing the number of synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1.01
[0093](3R,4R)-1-[(9-Deazahypoxanthin-9-yl)methyl]-3-hydroxy-4-hydroxymethyl-pyrrolidine (4). Starting with 9-deazahypoxanthine (Fumeaux and Tyler, J. Org. Chem., 1999, 64, 8411-8412) and (3R,4R)-3-hydroxy-4-hydroxymethyl-pyrrolidine hydrochloride (5) (Evans et al, J. Med. Chem., 2003, 46 5271-5276), the Mannich reaction general procedure (above) was followed to afford compound 4 as the acetic acid salt. After conversion to the HCl salt and 1H and 13C NMR spectra analysis, the compound was found to be identical in all respects with that previously reported (Evans et a.l J. Med. Chem. 2003, 46, 5271-5276).
example 1.02
[0094](3R,4R)-1-[(9-Deaza-adenin-9-yl)methyl]-3-hydroxy-4-(hydroxymethyl)-pyrrolidine (6). Starting with 9-deaza-adenine (Preparative Example 3.01) and (3R,4R)-3-hydroxy-4-hydroxymethyl-pyrrolidine (5), the Mannich reaction general procedure (above) was followed to afford compound 6 as the acetic acid salt. 1H NMR (d4-MeOH) δ 8.20 (s, 1H), 7.65 (s, 1H), 4.27 (s, 1H), 4.22 (quintet, J=3.0 Hz, 1H), 3.59 (m, 2H), 3.46 (dd, J=11.1, 8.3 Hz, 1H), 3.26 (dd, J=11.4, 5.7 Hz, 1H), 3.11 (dd, J=11.4, 3.0 Hz, 1H), 2.95 (dd, J=11.2, 6.8 Hz, 1H), 2.37 (brs, 1H), 1.82 (s, 3H). 13C NMR (d4-MeOH) 152.9, 151.9, 147.1, 132.0, 115.8, 108.2, 73.6, 63.1, 61.9, 56.0, 50.8, 49.5, 23.7. HRMS (MH+) calc for C12H18N5O2: 264.1461. Found 264.1457.
example 1.03
[0095](3R,4S)-4-(Benzylthlomethyl)1-[(9-deaza-adenin-9-yl)methyl]-3-hydroxy-pyrrolldine (7). The Mannich reaction general procedure (above) was followed to afford compound 7 as the acetic acid salt. The acetic acid salt was converted to the free base via ion exchange chromatography. 1H NMR (d4-MeOH) 8.17 (s, 1H), 7.46 (s, 1H), 7.26-7.16 (m, 5H), 3.93-3.90 (m, 1H), 3.83-3.74 (m, 2H), 3.68 (s, 2H), 3.03-2.97 (m, 1H), 2.80 (dd, J=10.2, 6.4 Hz, 1H), 2.66-2.58 (m, 2H), 2.38 (dd, J=12.5, 8.9 Hz, 1H), 2.30 (dd, J=9.5, 7.2 Hz, 1H), 2.20-2.14 (m, 1H). 13C NMR (d4-MeOH) 152.5, 151.4, 147.4, 140.4, 130.4, 130.4, 129.8, 115.5, 112.9, 77.3, 62.7, 59.2, 49.3, 48.6, 37.5, 35.6. HRMS (MH+) calc for C19H24N5OS: 370.1702. Found 370.1694.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com