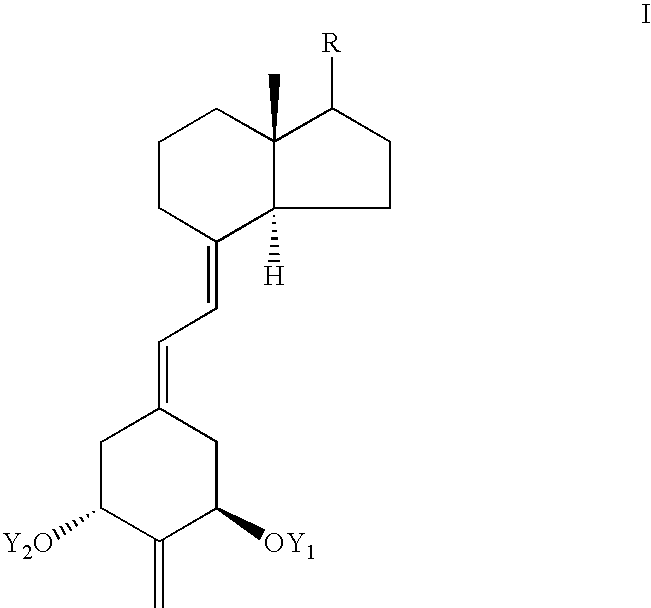
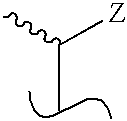
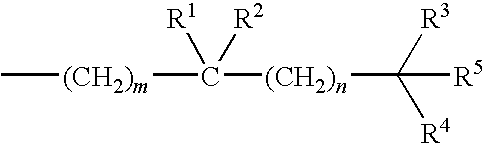
Treatment of Inflammatory Bowel Disease With 2-Methylene-19-Nor-Vitamin D Compounds
a technology of vitamin d and compound, which is applied in the field of vitamin d compounds, can solve the problems of dietary intake of vitamin d, decreased bone mineral density, and hypercalcemia, and achieve the effect of preventing inflammatory bowel diseases and/or preventing ibd
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0062]Recently a number of transgenic animals have been developed in which IBD symptoms occur spontaneously. One of the best animal models for Crohn's disease is the IL-10 knockout (KO) mouse (Kuhn et al. 1993, Mac Donald 1994). In conventional animal facilities, the IL-10 KO mice develop enterocolitis within 5-8 weeks of life (Kuhn et al. 1993). Approximately 30% of the IL-10 KO mice die following the development of severe anemia and weight loss (Kuhn et al. 1993). The enterocolitis which develops in IL-10 KO mice is due to an uncontrolled immune response to conventional microflora since germfree IL-10 KO mice do not develop disease. In addition mice raised in specific pathogen free facilities develop milder disease which doesn't result in the death of the mice (Kuhn et al. 1993). There are limitations involved in studying IL-10 KO mice as a model of IBD. If vitamin D is a regulator of IL-10 production then the results in this animal model may not represent 320 what may happen in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com