Antibacterial and antifungal peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
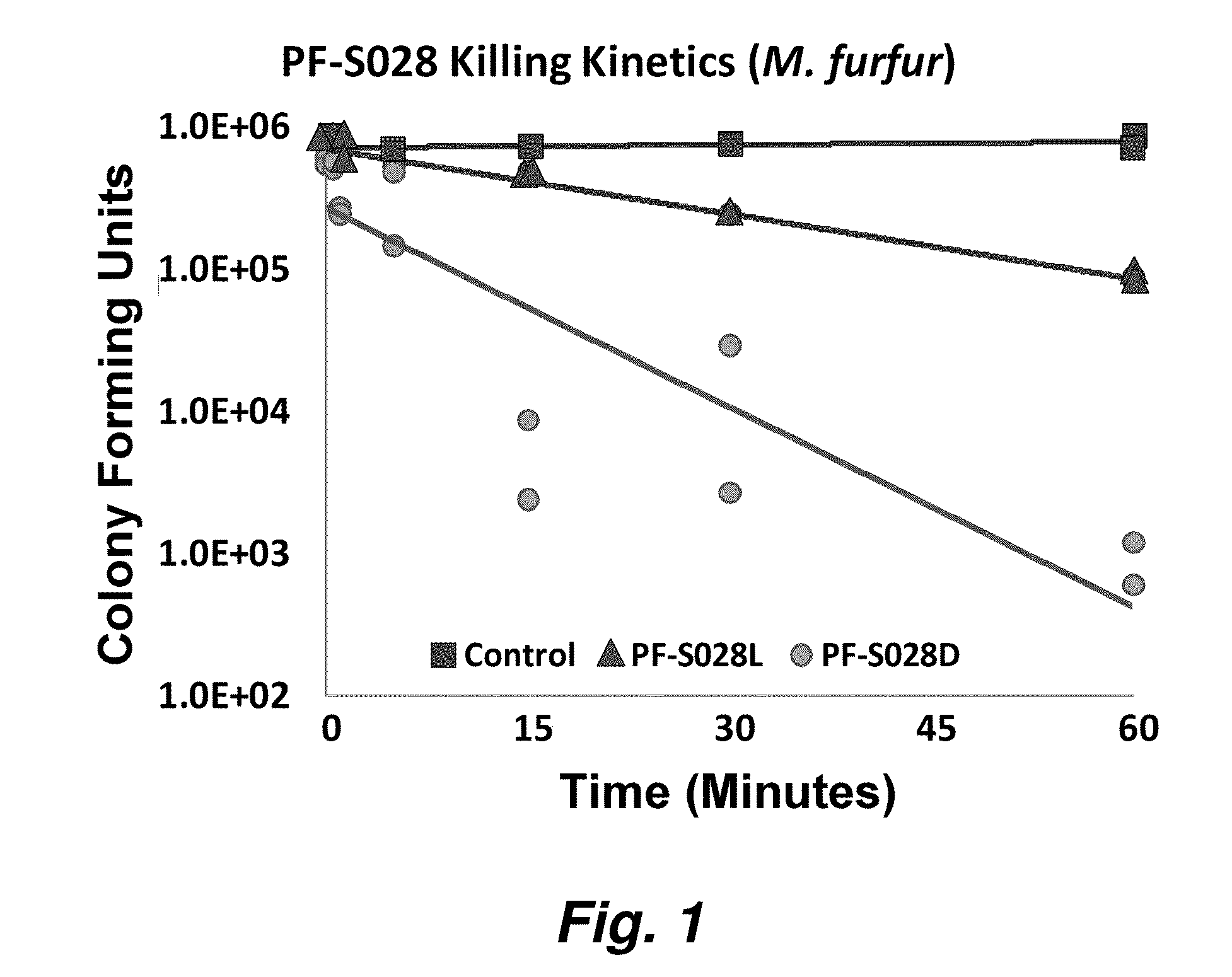
Image
Examples
Example
Example 1
Synthesis and Screening of Antimicrobial Peptides
[0221]Peptides were synthesized utilizing standard solid-phase synthesis methods (Fmoc or Boc chemistries). In certain embodiments, peptides were synthesized using Fmoc (9-fluorenylmethoxy carbonyl) solid-phase synthesis utilizing single or double coupling cycles at 0.01 to 0.25 mmol scales. Briefly, N-terminal deprotection was conducted in 20% (v / v) piperidine / N-methylpyrrolidone (NMP) for 3 min followed by eight washes with NMP. For the coupling cycle, amino acids were solubilized in 0.45 M N-hydroxybenzotriazole (HOBt) / HBTU (O-benzotriazole-N,N,N,N-tetramethyl-uronium hexafluoro-phosphate) in dimethylformamide (DMF) with 0.9 M diisopropyl ethylamine (DIEA) (1:1:1:2 ratio Fmoc amino acid:HOB:HBTU:DIEA) before being added to the resin for 30 min, followed by 10 NMP rinses. As needed, peptides were then labeled N-terminally with 4-molar excess fluorescent dyes or blocking groups in HOBt / HBTU / DIEA coupling solution with shakin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com