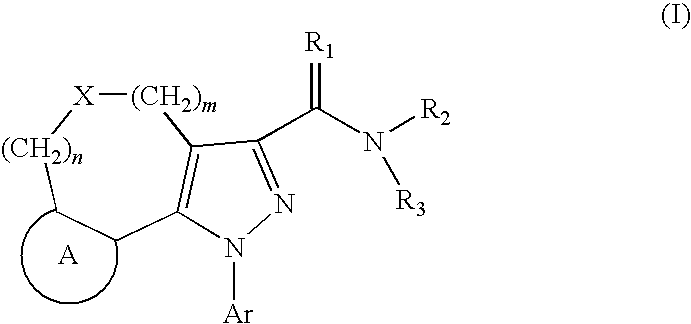
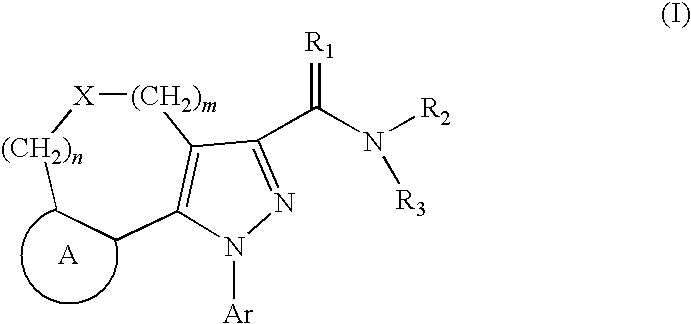
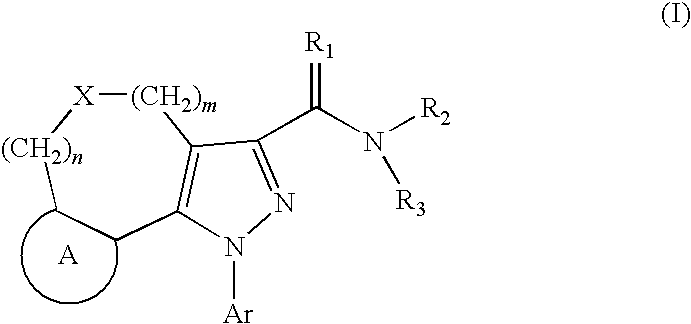
Tricyclic pyrazole derivatives as cannabinoid receptor modulators
a technology of cannabinoid receptor and tricyclic pyrazole, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problem of few medicines availabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-Chloro-N-{[8-chloro-1-(2,4-dichloro-phenyl)-1,4,5,6-tetrahydro-1,2-diaza-benzo[e]azulene-3-yl]methylamino-methylene)-benzenesulfonamide
[0103]8-chloro-1-(2,4-dichloro-phenyl)-1,4,5,6-tetrahydro-1,2-diaza-benzo[e]azulene-3-carboxylic acid was prepared by suitable modifications of similar techniques and processes known in the art.
Step A
4-Chloro-N (8-chloro-1-(2,4-dichloro-phenyl)-1,4,5,6-tetrahydro-1,2-diaza-benzo[e]azulene-3-carbonyl)-benzene-sulfonamide
[0104]8-Chloro-1-(2,4-dichloro-phenyl)-1,4,5,6-tetrahydro-1,2-diaza-benzo[e]azulene-3-carboxylic acid (4.3 g, 10.55 mmol) was converted into acid chloride using thionyl chloride (2.31 mL, 31.66 mmol) in toluene by refluxing at ca. 110° C. over a period of 1 h.
[0105]The solvents were evaporated under reduced pressure. The residue obtained was taken in dichloromethane (20 mL) and the resulting solution was added to the cooled suspension of 4-chloro benzene sulfonamide (2.7 g, 4.137 mmol) and TEA (1.97 mL, 14.137 mmol) in dichloromethan...
example 2
Hydrochloride salt of 8-chloro-1-(2,4-dichloro-phenyl)-4,5-dihydro-1H-6-oxa-1,2-diaza-benzo[e]azulene-3-carboxylic acid piperidin-1-ylamide
[0110]a) 8-Chloro-1-(2,4-dichloro-phenyl)-4,5-dihydro-1H-6-oxa1,2-diaza benzo[e]azulene-3-carboxylic acid by suitable modifications of similar techniques and processes known in the art.
[0111]b) Hydrochloride salt of 8-Chloro-1-(2,4-dichloro-phenyl)-4,5-dihydro-1H-6-oxa 1,2-diaza benzo[e]azulene-3-carboxylic acid piperidin-1-yl-amide (Compound 2). 8-chloro-1-(2,4-dichloro-phenyl)-4,5-dihydro-1H-6-oxa 1,2-diaza-benzo[e]azulene-3-carboxylic acid (0.500 g, 1.221 mmol) was coupled with 1-amino-piperidine (0.197 mL, 1.832 mmol) in presence of 1-Hydroxy-benzotriazole monohydrate (HOBt. H2O), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC-HCl) and TEA in dichloromethane (25 mL) at ca. 27° C. over a period of 25-30 min. The reaction was diluted with H2O (30 mL) and extracted with dichloromethane. The dichloromethane layer was separated, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lipophilicity | aaaaa | aaaaa |
| metabolic stability | aaaaa | aaaaa |
| valence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com