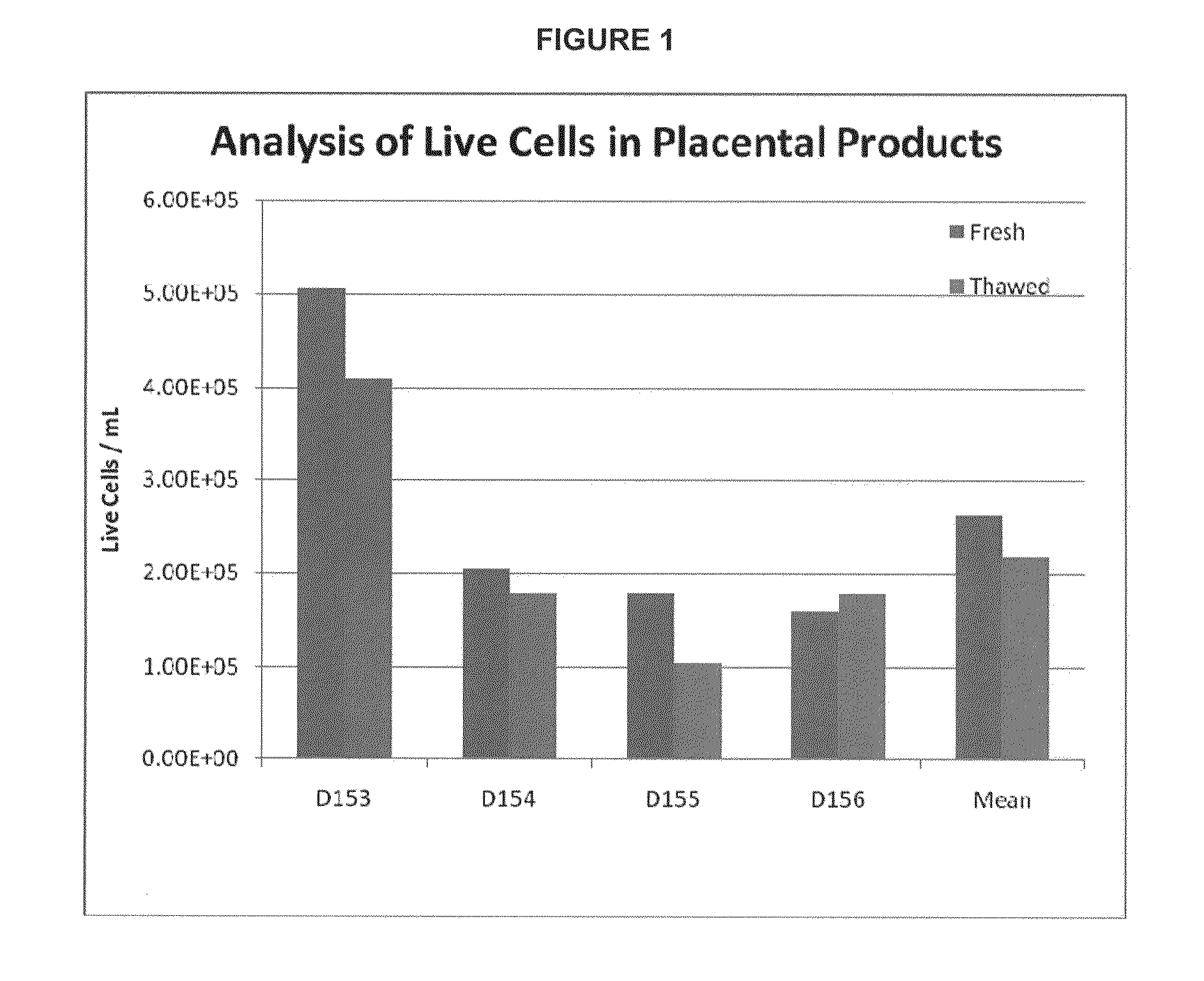
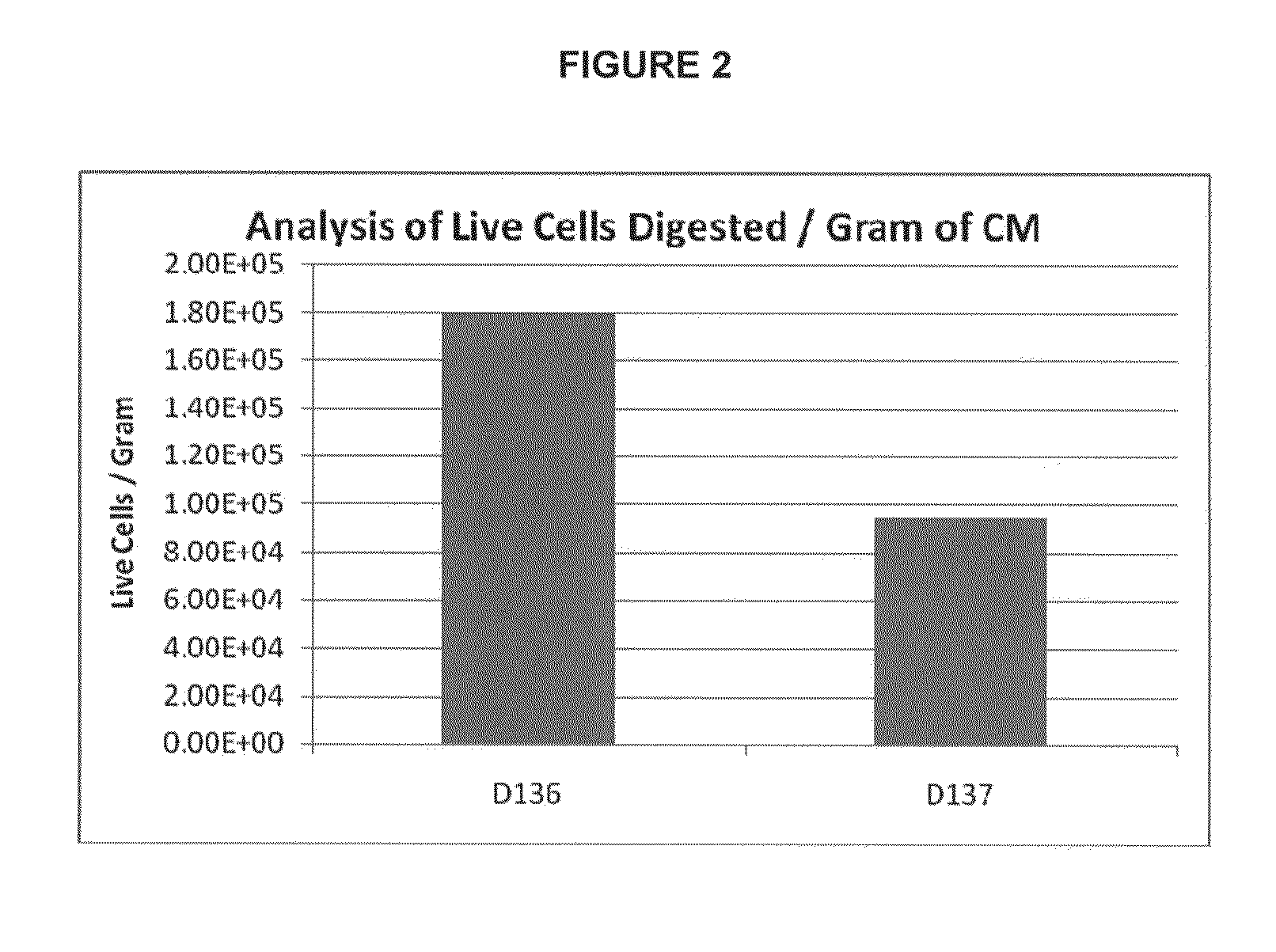
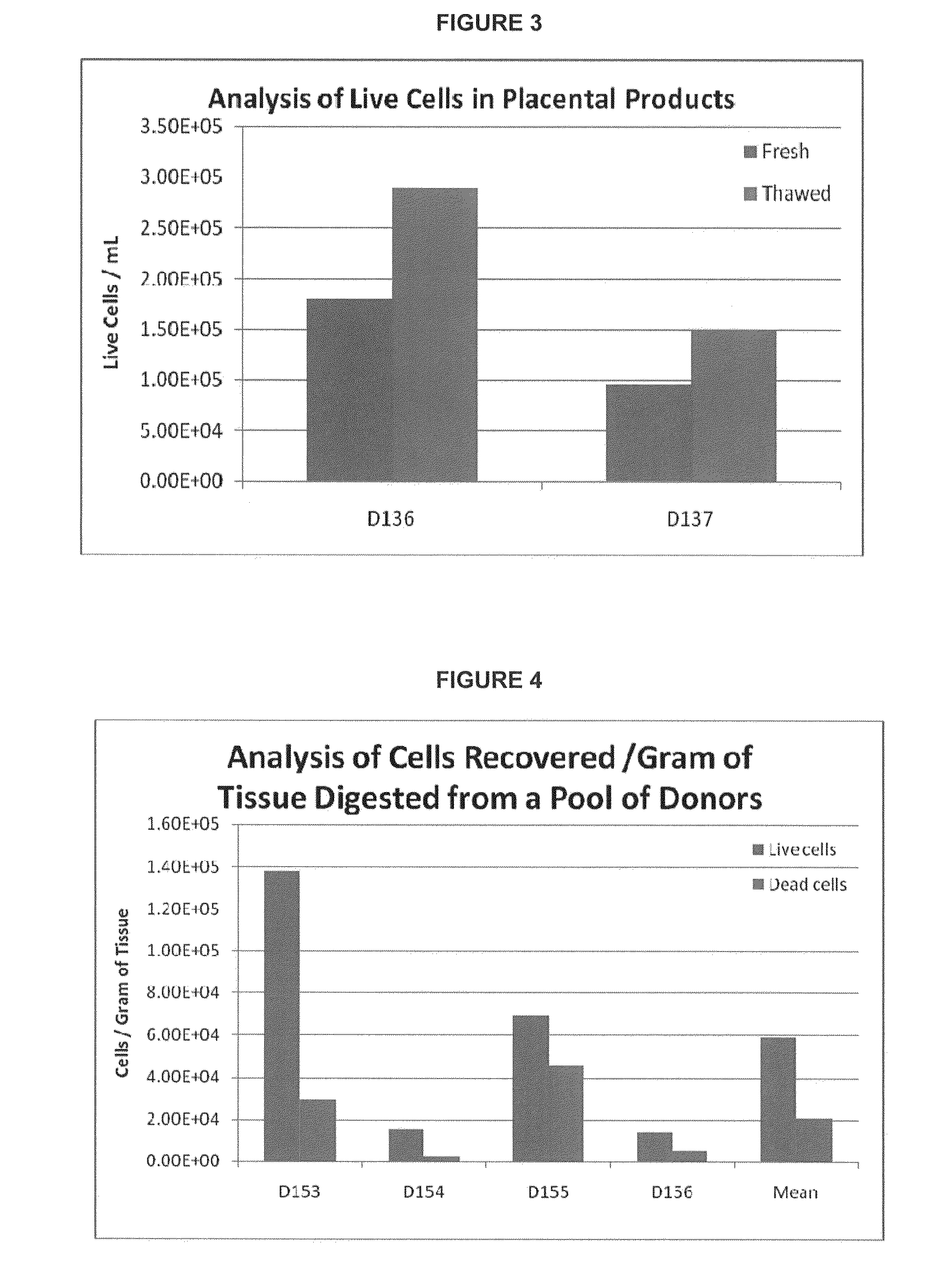
Methods of manufacture of therapeutic products comprising vitalized placental dispersions
a technology of vitalized placental dispersions and placental products, which is applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of high morbidity and mortality rate, and insufficient treatment, and achieves the effect of limited digestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Obtaining Placental Tissue
[0176]A whole placenta was obtained from a registered tissue bank after informed consent. The placenta and placed, with the maternal surface (rough surface) face down, on a sterile tray. The amniotic-chorionic membrane was cut and removed from the placenta. The chorionic membrane was then separated from the amnion and washed twice in PBS.
[0177]The chorionic membrane was then soaked in an anticoagulant (ACD-A) solution to remove blood clots and then washed again in PBS.
[0178]The chorionic membrane was then digested by incubation with dispase II for 30 min. at 37° C. The trophoblast layer was mechanically removed by scraping with fingers and the chorion was washed again in PBS.
[0179]The chorionic membrane was then incubated for 24 hours in an antibiotic cocktail containing gentamicin, vancomycin, and amphotericin B, and washed again in PBS.
example 2
Digesting Placental Tissue
[0180]A chorion membrane (obtained from Example 1) was digested by incubation in 200 mL of a collagenase II solution (300 U / mL in DMEM) for 10 min at 37° C. The chorionic membrane was then removed, leaving a digestion suspension containing collagenase and placental cells.
[0181]The volume and container for digestion was determined based on the need to provide a suitable digestion environment for the tissue once placed on a shaker. The digestion was carried out on a standard plate shaker set at moderate speed in a 37° C. cell culture incubator.
example 3
Obtaining Placental Cells
[0182]A digestion suspension comprising placental cells (obtained from Example 2) was centrifuged at 2000 rcf for 5 min to separate the digestive enzyme (collagenase II) from the placental cells. This step centrifugation step may enhance cell viability by preventing over-digestion and ensure that the enzyme is washed away before homogenizing the tissue. This centrifugation step pellets the cells without damaging them, allowing the collagenase II to be removed as supernatant.
[0183]The cells were then centrifuged again, the supernatant poured off, and the placental cells were resuspended in a small volume (2 mL) of cryprotectant (5% DMSO in saline). Two mL provides an adequate volume to resuspend the cells while not over-diluting the chorion membrane dispersion once the cells have been added.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com