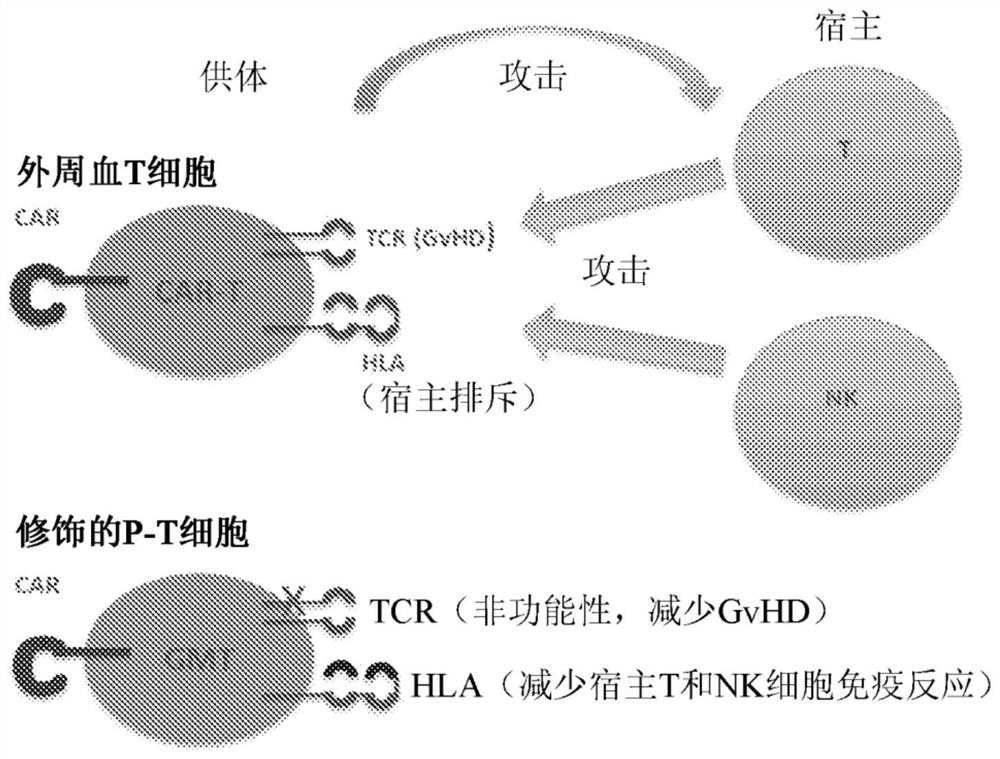
Placenta-derived allogeneic car-t cells and uses thereof
A cell and placental technology, applied in embryonic cells, animal cells, receptors/cell surface antigens/cell surface determinants, etc., can solve problems such as reduced alloreactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
example 1
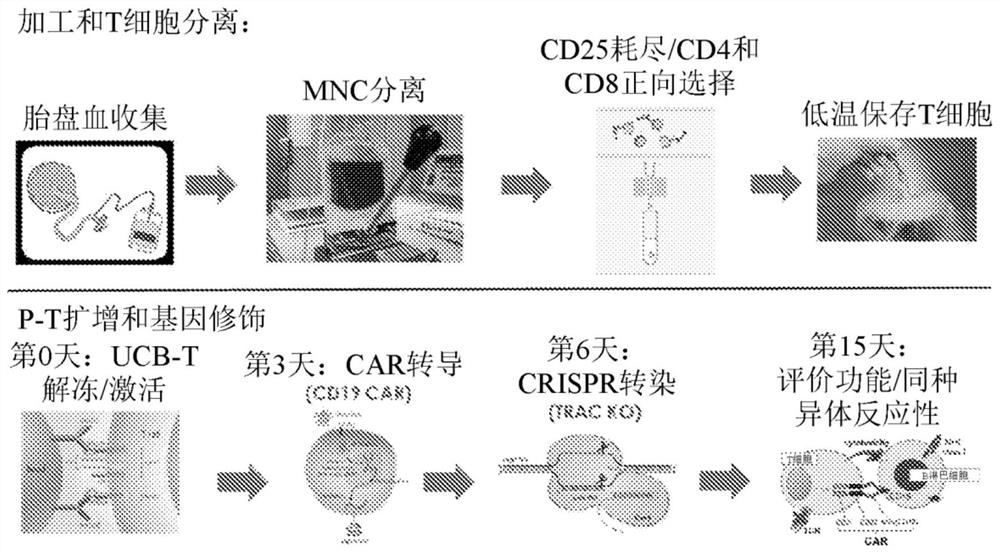
[0082] Example 1: Starting material, MNC isolation and T cell isolation
[0083] The starting material placental blood (which contains human umbilical cord blood (UCB) and / or human placental perfusate (HPP)) was collected by LifebankUSA with informed consent. Following collection, mononuclear cells (MNCs) were enriched in the starting material using Hetastarch RBC sedimentation or Ficoll-Paque density gradient cell separation. Next, MNCs undergo a forward selection process to deplete CD25+ T regulatory T cells (Treg) followed by forward selection against CD4+ and CD8+ T cells using the Militenyi Bead Cell Isolation Kit. Aliquots of isolated T cells were obtained for serological and sterility testing, as well as phenotypic analysis, prior to freezing the cells.
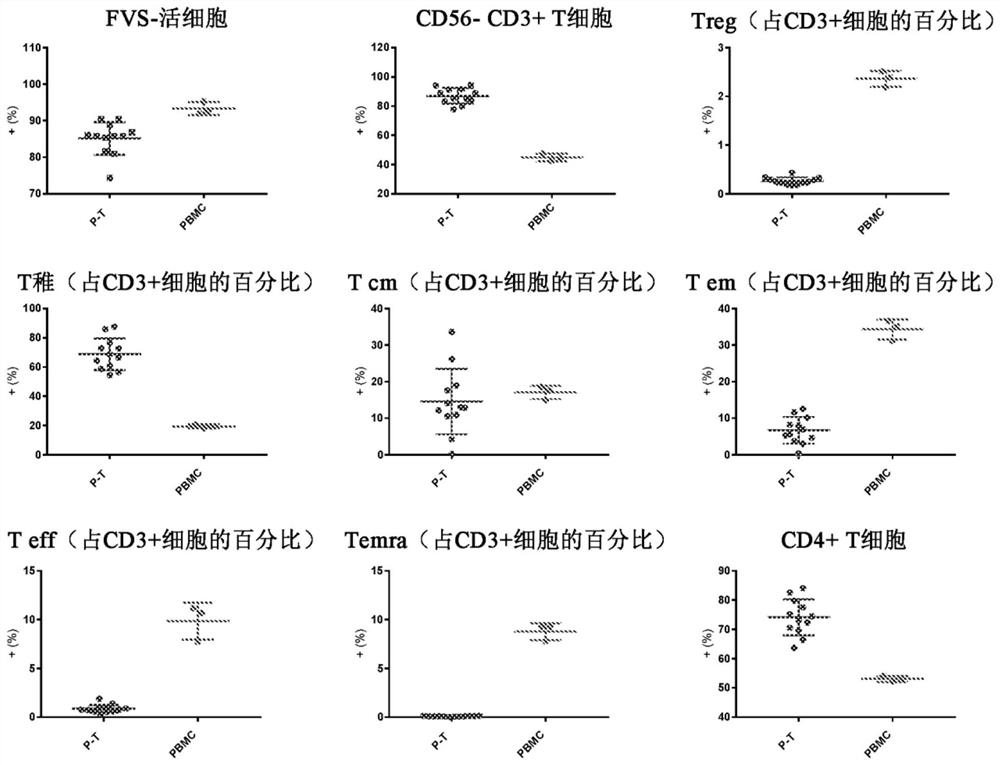
[0084] The phenotype of isolated P-T cells differs from peripheral blood mononuclear cells (PBMC). P-T cells contained >78% CD3+CD56- T cells and consisted primarily of CD3+CD45RA+CCR7+CD27+ naive T cells with a lo...
example 2
[0087] Example 2: T cell activation and expansion
[0088] Unmodified P-T cells:
[0089] The isolated P-T cells were thawed, subjected to CD25 depletion using Miltenyi anti-CD25 beads to remove CD4+CD25+CD127-Tregs (which can be included prior to the T cell isolation step), and treated with anti-CD3 / anti-CD28 Dynabeads from Invitrogen ( 1:1 bead:cell ratio) or activated with anti-CD3 / anti-CD28 nanoparticles Transact (1:100 volume dilution) from Miltenyi. Next, cells were expanded with 100 IU / mL IL-2, 10 ng / mL IL-7 + 10 ng / mL IL-15, or 100 IU / mL IL-2 + 10 ng / mL IL-7. Additional restimulations were done on days 12-14, and cells were expanded in Grex vessels until day 21 to maximize fold expansion.
[0090] When cultured to day 20, unmodified P-T cells can expand up to 600-fold on initial stimulation and up to 3,600-fold on restimulation (RS) on day 14.
[0091] Under various culture conditions, unmodified P-T expanded for 20 days exhibited an earlier differentiated phenoty...
example 3
[0102] Example 3: CD19 CAR and CD20 CAR in vitro activity
[0103] Day 15 Cytolytic activity of P-CD19 CAR-T cells against cancer cell lines
[0104] On days 2-4 of UCB-T culture, activated UCB-T cells were transduced with CD19 CAR retrovirus or lentivirus using centrifugation inoculation. CAR expression was detected using a FITC-labeled recombinant CD19-Fc fusion protein, or an anti-Myc PE antibody if the CAR vector contained a Myc tag. UCB-CAR-T activity was assessed using the following two assays.
[0105] CD19 CAR-transduced UCB-T cells specifically killed CD19+ Daudi cancer targets at levels comparable to PBMC CD19CAR T cells, but not CD19-K562 cells.
[0106] CD20 CAR-transduced UCB-T cells specifically killed CD20+ Daudi cancer targets at levels comparable to PBMC CD20 CAR T cells, but not CD20-Molp8 cells.
[0107] CD19 CAR-transduced UCB-T cells specifically secreted the proinflammatory cytokines IFN-g and GM-CSF and secreted the cytolytic effector protein pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com