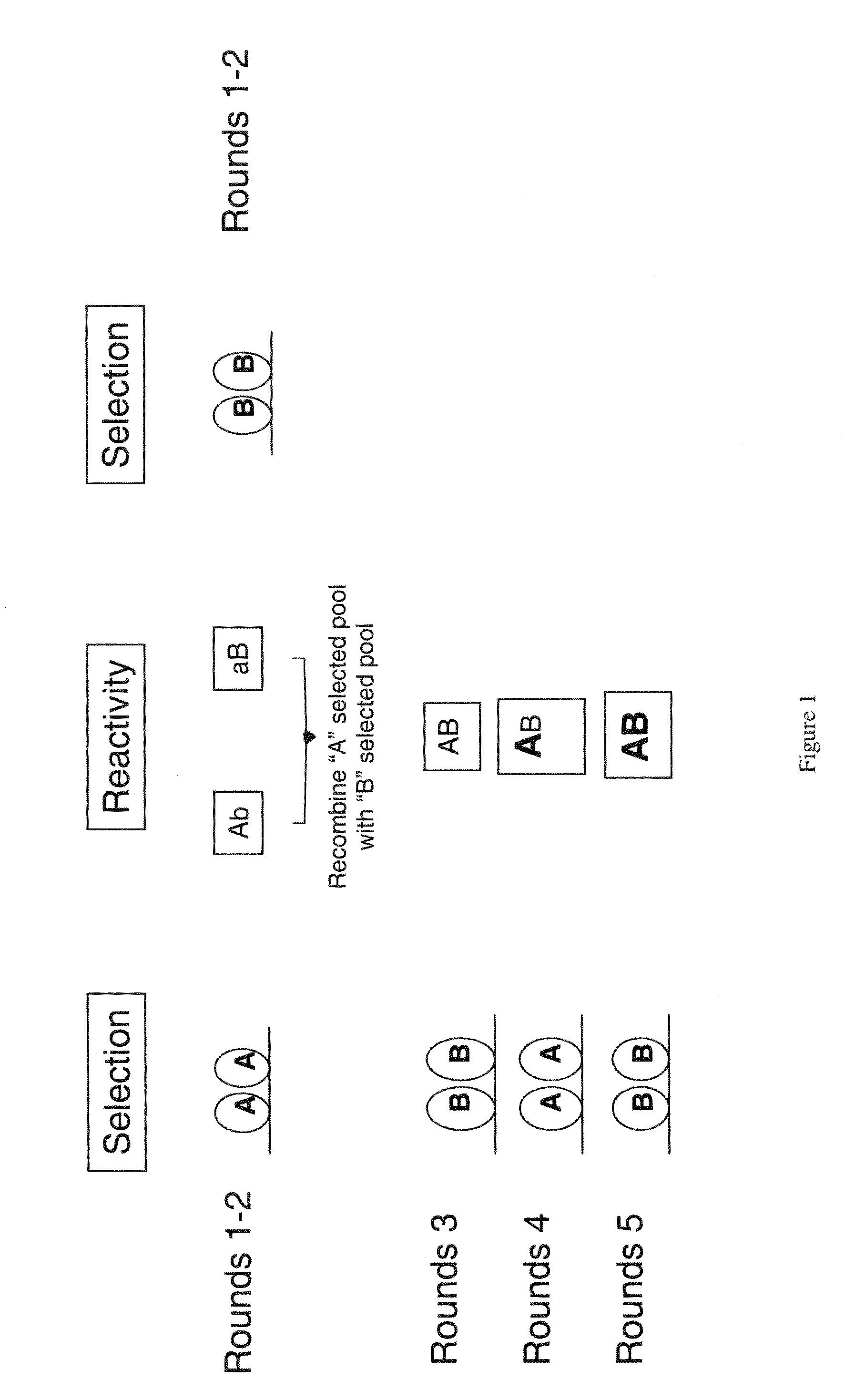
Neutralizing molecules to influenza viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antibody Libraries from Influenza Donors
[0217]Donors selected for inclusion into the Comprehensive Influenza Library were confirmed to have had a previous influenza infection, been approximately 5 years old at the time of a the 1957 H2N2 or 1968 H3N2 influenza pandemics, and to be in current good health. Serology on a panel of H1N1 A / NewCaledonia / 20 / 99, H3N2 A / Panama / 2007 / 99 and H5N1 A / Vietnam / 1203 / 2004 virus or hemagglutinin proteins was performed to confirm the presence of antibodies to the hemagglutinin proteins.
[0218]First 5-20 ml of bone marrow was collected from each donor meeting the selection criteria and mixed with RNA later (Applied Biosystems) per the manufacturer's instructions to preserve the integrity of cellular RNA. RNA was isolated using a TRI-BD reagent protocol (Sigma-Aldrich).
[0219]Heavy chain and light chain repertoires were recovered from each donor derived RNA by RT-PCR using random primed cDNA template for heavy chains, oligo dT primed cDNA template for light...
example 2
Preparation of Neutralizing Antibodies
[0223]Antibodies derived from human bone marrow phage display antibody libraries (see Example 1) were converted and tested as mammalian expressed immunoglobulins, as previously described (see also, Kashyap A K et al., Proc Natl Acad Sci USA. 2008 Apr. 22; 105(16):5986-91). The heavy chains fell into two sequence classes:
(SEQ ID NO: 1)QVQLQESGGGLVQPGESLRLSCVGSGSSFGESTLSYYAVSWVRQAPGKGLEWLSIINAGGGDIDYADSVEGRFTISRDNSKETLYLQMTNLRVEDTGVYYCAKHMSMQQVVSAGWERADLVGDAFDVWGQGTMVTVSS(SEQ ID NO: 2)QVQLQQSGPRLVKPSQTLSLTCAISGDSVSGDSGTWNWIRQSPSRGLEWLGRTYYRSKWYNDYAESVKSRIVIKADTSKNEFSLQLNSVTPEDTAIYYCARAGVKIFGLIVGALDNWGRGTLVTVSS
[0224]The underlined hypervariable CDR regions are shown for the heavy chains as follows.
(SEQ ID NO: 7)GESTLSYYAVS(SEQ ID NO: 8)WLSIINAGGGDID(SEQ ID NO: 9)AKHMSMQQVVSAGWERADLVGDAFD(SEQ ID NO: 10)SGDSGTWN(SEQ ID NO: 11)WLGRTYYRSKWYND(SEQ ID NO: 12)ARAGVKIFGLIVGALD
[0225]The heavy chain described by SEQ ID NO:1 was found to pair with one lambda ...
example 3
Generating Universal Influenza Vaccines
[0233]The goal of vaccine design against heterogeneous pathogens is to identify and design effective and broadly protective antigens. In the case of influenza, considerable historical efforts have gone into the empirical testing of conserved linear sequences and regions with little success. A plausible reason for these failures is a lack of knowledge that focused responses against antigenic test articles are actual bona fide productive sites for neutralization of an antigen on the pathogen in the setting of an actual infection. For influenza one would be expect to find these bona fide solutions within the repertoires of survivors of an influenza infection. In our case we have demonstrated that certain antibodies amongst a large collection of antibodies are capable of neutralizing multiple subtypes of Influenza. Some of these antibodies neutralize influenza through classical inhibition of hemagglutination. Collectively, we expect that the design...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com