Sleeves for expandable medical devices
a technology of medical devices and sleeves, applied in the field of transcatheter delivery and remote deployment of implantable medical devices, can solve the problems of constrained stent graft positioning, difficult to remove or expand sleeves, and sleeves may give rise to other problems or issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016]While the present disclosure describes a number of embodiments, it will be understood that the disclosure is not limited to these embodiments. Instead, it is intended to cover all alternatives, modifications, and equivalents as may be included within the spirit and scope of the disclosure as described and claimed.
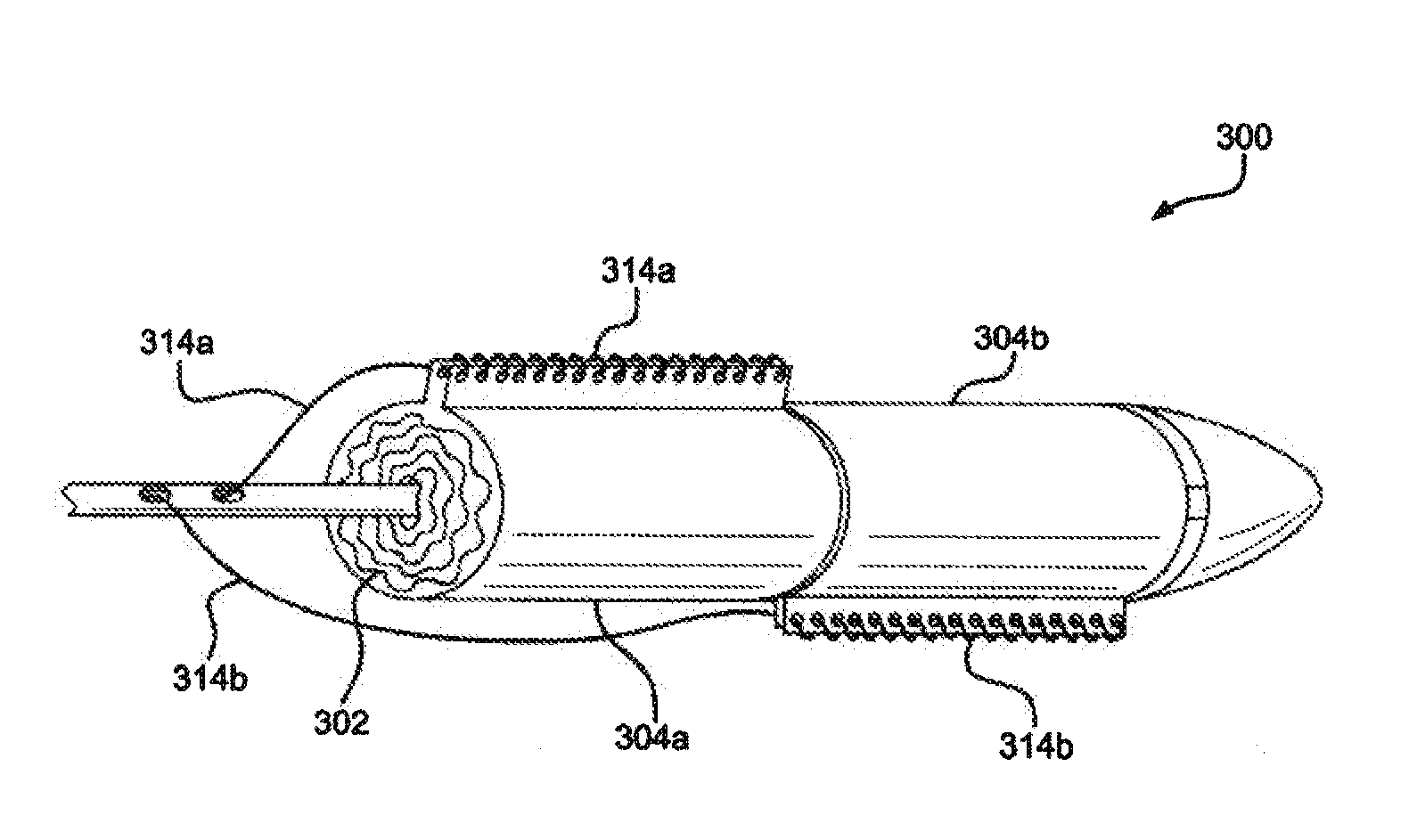
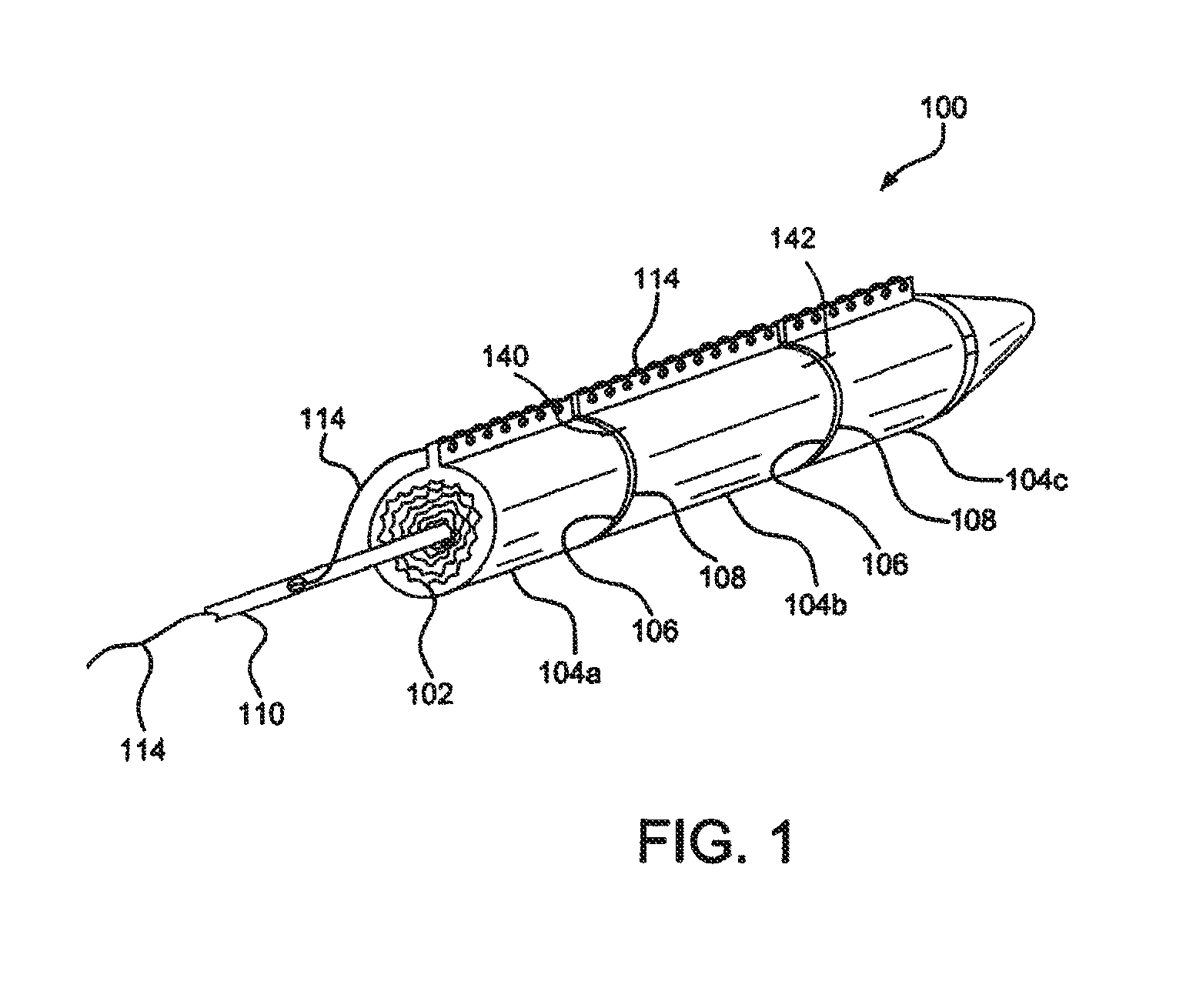
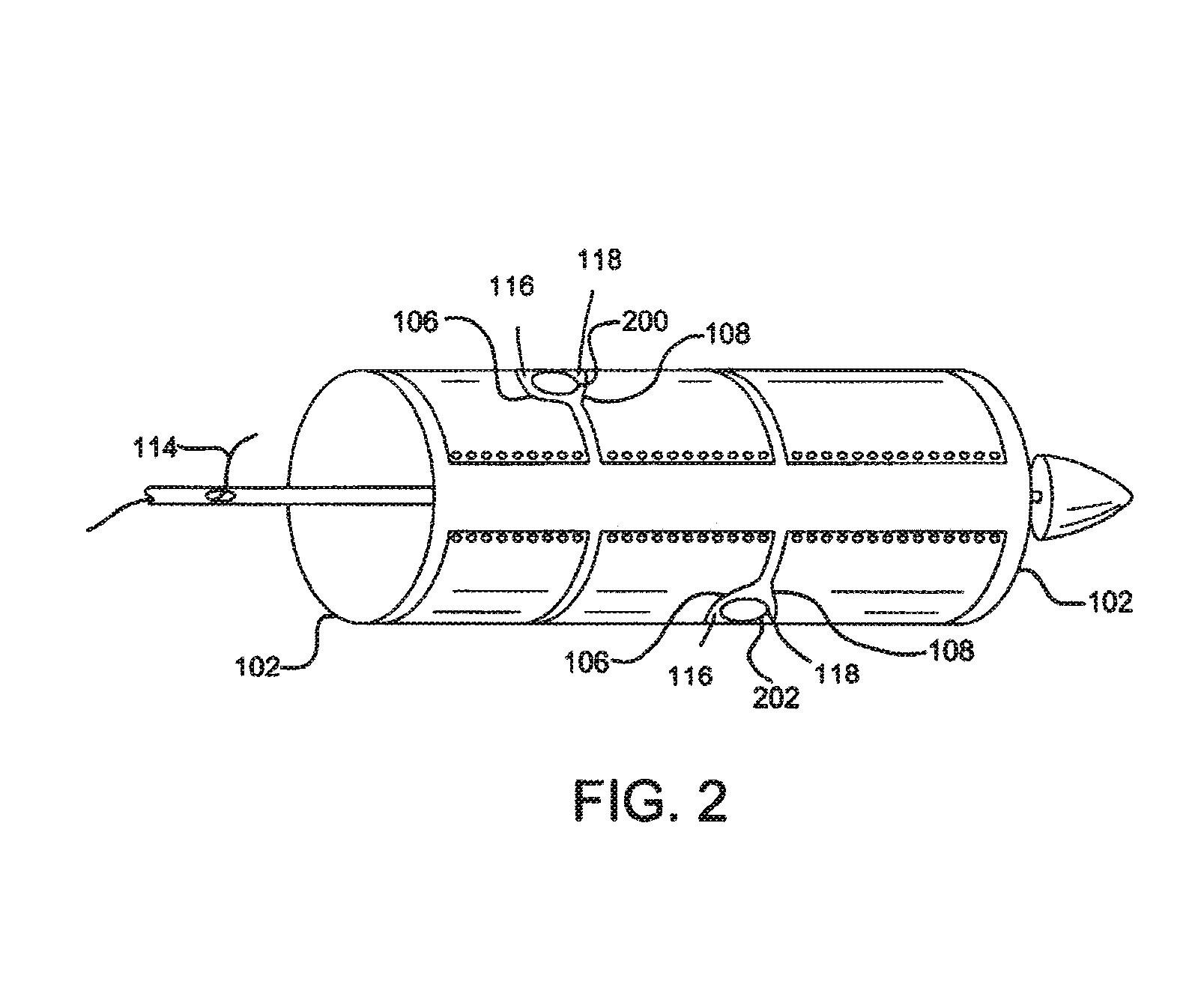
[0017]Various embodiments of the present disclosure comprise a catheter assembly configured to deliver an expandable implant to a treatment area of the vasculature of a patient. In accordance with embodiments of the disclosure, an expandable implant is constrained by one or more sleeves. When the sleeves are expanded or removed, one or more target portions of the expandable implant can be exposed. In various embodiments, the target portion or portions comprise side branch fenestrations or fenestratable portions. Such target portions may be revealed between adjacent sleeves or between the edges of a single sleeve.
[0018]With initial reference to FIG. 1, a catheter assem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com