Compounds and assays for controlling Wnt activity
a technology of compound and assay, applied in the field of therapeutic methods, can solve the problems of impaired glucose tolerance when challenged, impaired glucose-induced insulin secretion from the pancreatic islets, and impaired glucose toleran
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Virtual Screening
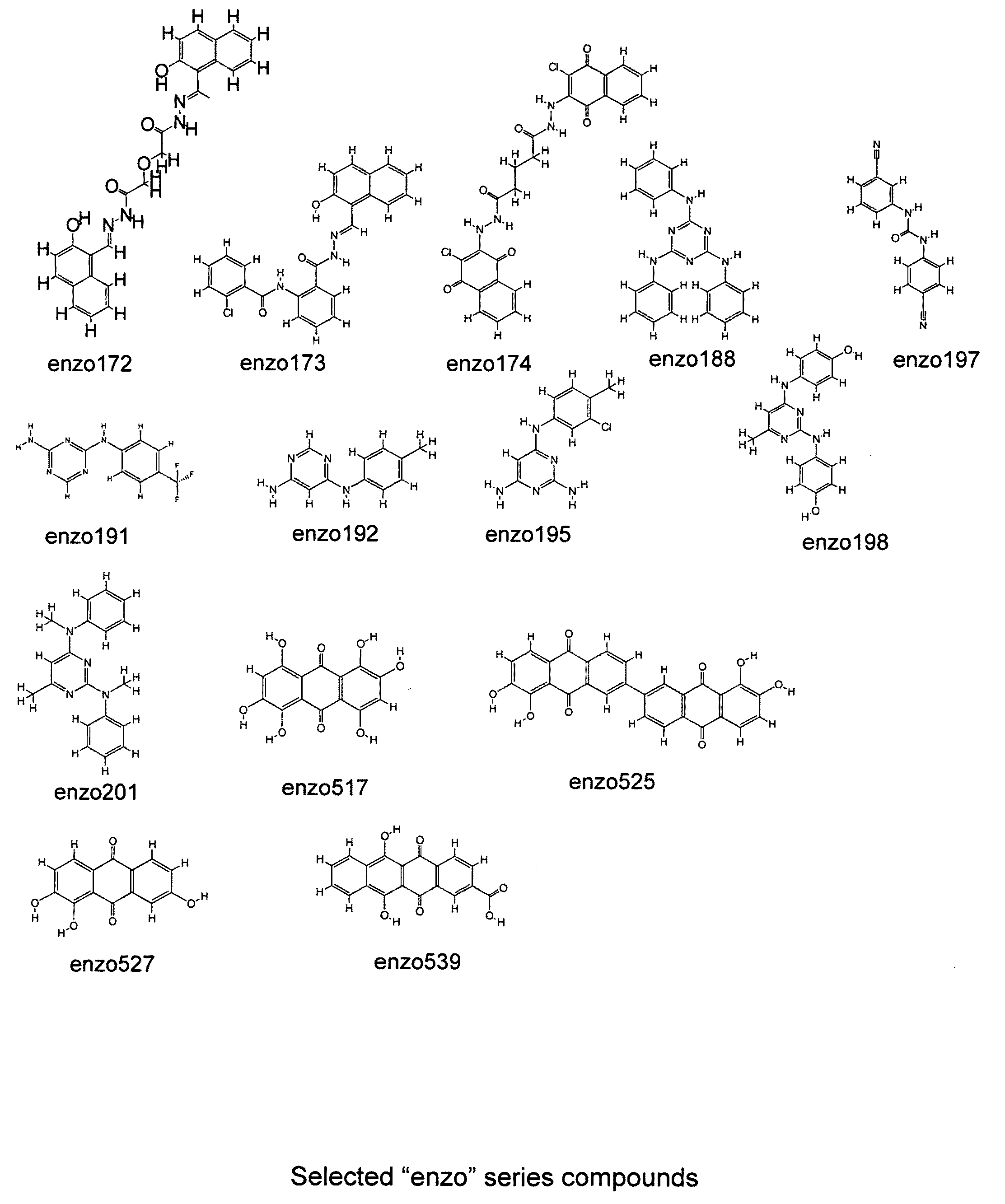
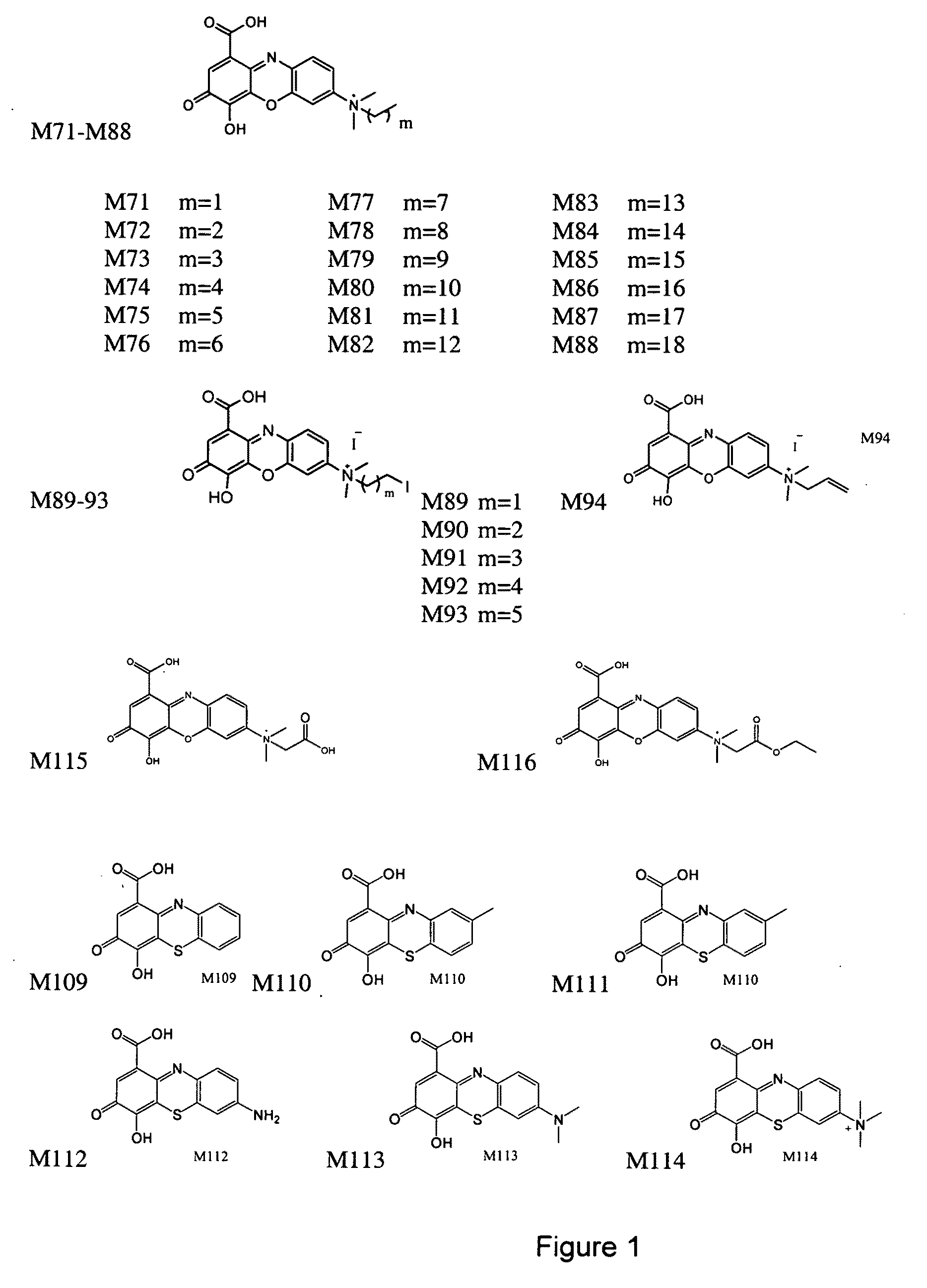
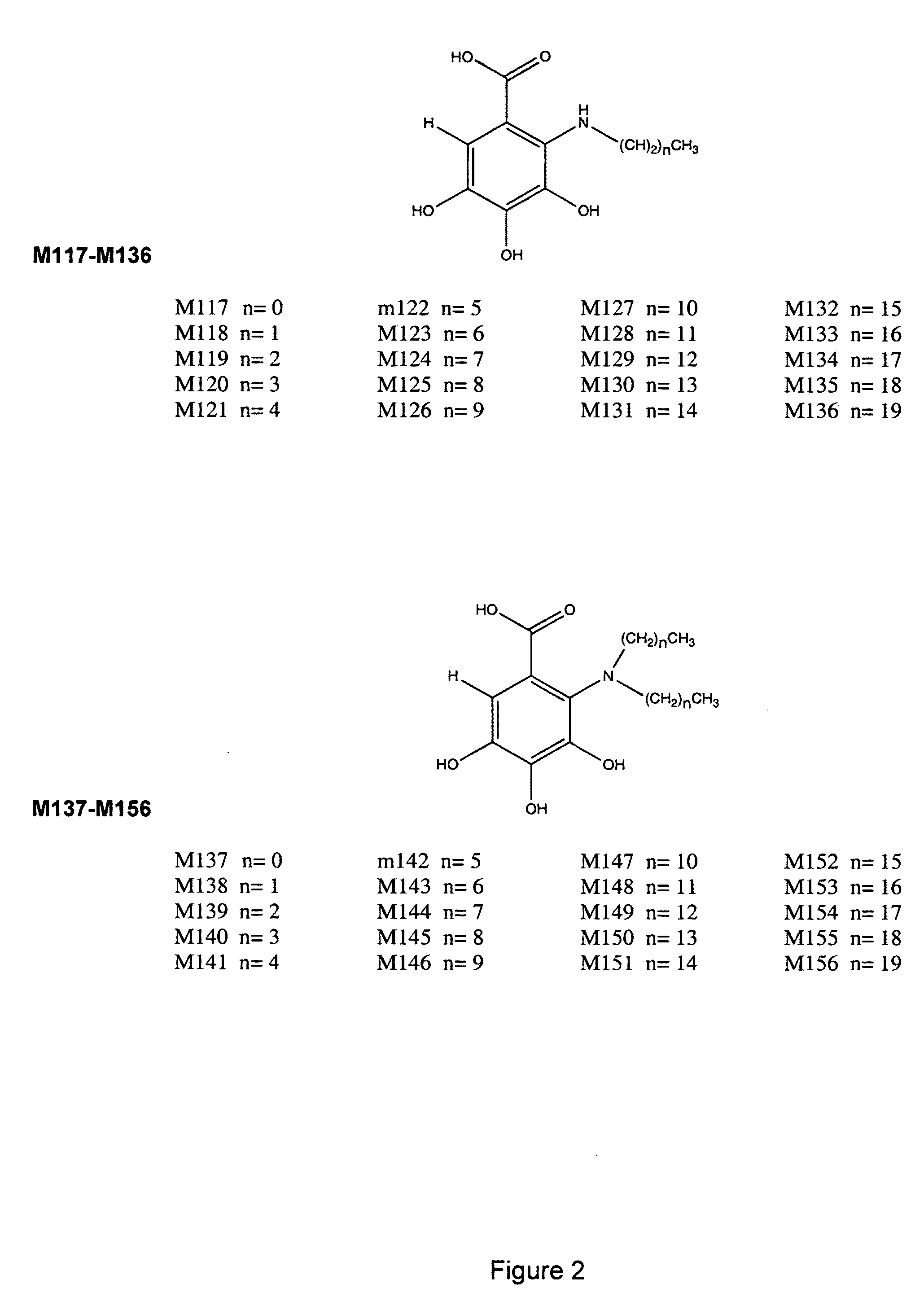
[0167]The designs of the various compounds that comprise a virtual library are shown in FIGS. 1-13 and are designated as compounds M71-M364. Another series of compounds were derived from the NCl database / chemical library as well as commercial database / chemical libraries from ChemDiv, Inc. (San Diego, Calif.), Chembridge Corporation (San Diego, Calif.) and Life Chemicals USA (Orange, Conn.). This series is referred to as Enzo001-Enzo438. Compounds derived from the M71-M364 series are novel compounds of a virtual library that require synthesis before testing in a biological assay. On the other hand, the Enzo001-Enzo438 series represents a physical library—a series of compounds that have already been synthesized and are readily available for testing. These compounds were screened as described previously in related pending U.S. patent application Ser. No. 10 / 849,067 and candidates were selected on the basis of a predicted binding to Domain III of LRP5 / 6.
[0168]Although a...
example 2
Synthesis of Gallocyanine Analogues
[0169]One compound that has been of interest in the related patent applications is IIIC3, otherwise known as Gallocyanine and NCl 8642. A number of the compounds described in Example 1 are analogues of this compound and as described above, underwent a virtual screening step to select the best candidates. However, as mentioned in the text, analogues of compounds that show effectiveness may also be tested in the absence of such a screening step. Thus, a synthesis was carried out that produced at least two different IIIC3 analogues that were tested directly without the screening step. In this process, a mixture of 100 mg (0.33 mMoles) of gallocyanine (chloride free), 50 ml of methanol, 38 μl (0.33 mMoles) of 2,6-lutidine and propyliodide (0.5 ml) were placed in a 150 ml pressure vessel with a stirrer. The mixture was stirred and heated at approximately 110° C. for 4 days. After concentration in vacuo, the residue was washed with 5 ml of ethanol. Solid...
example 3
Effects of Gallocyanine Analogues on Mice Placed on a High Caloric Diet
[0170]This experiment was carried out essentially as described previously with IC15, IIIC3 and M01 in related pending U.S. patent application Ser. No. 11 / 598,916 with the exception of using the gallocyanine analogue preparation from Example 2 above. 8 week old C57 / BL6J mice were placed on a high fat diet for 60 days and then treated on a daily basis starting at day 50 with the M03 preparation from Example 2 (1 mg / kg / day) either by intraperitoneal injection or by oral gavage. On day 60, blood glucose levels were measured after overnight fasting. As shown in FIG. 14, with intraperitoneal administration, the control mice (N=15) had an average of 141.2 mg / dL of glucose while the mice (N=10) treated with the preparation from Example 2 had an average level of 116.8 g / dL. After administration by oral gavage, a similar effect was seen in the treated mice compared to the controls.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com