Method for extracting gallium from fly ash
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Example 1
[0065]The experimental procedures used in the example are as follows.
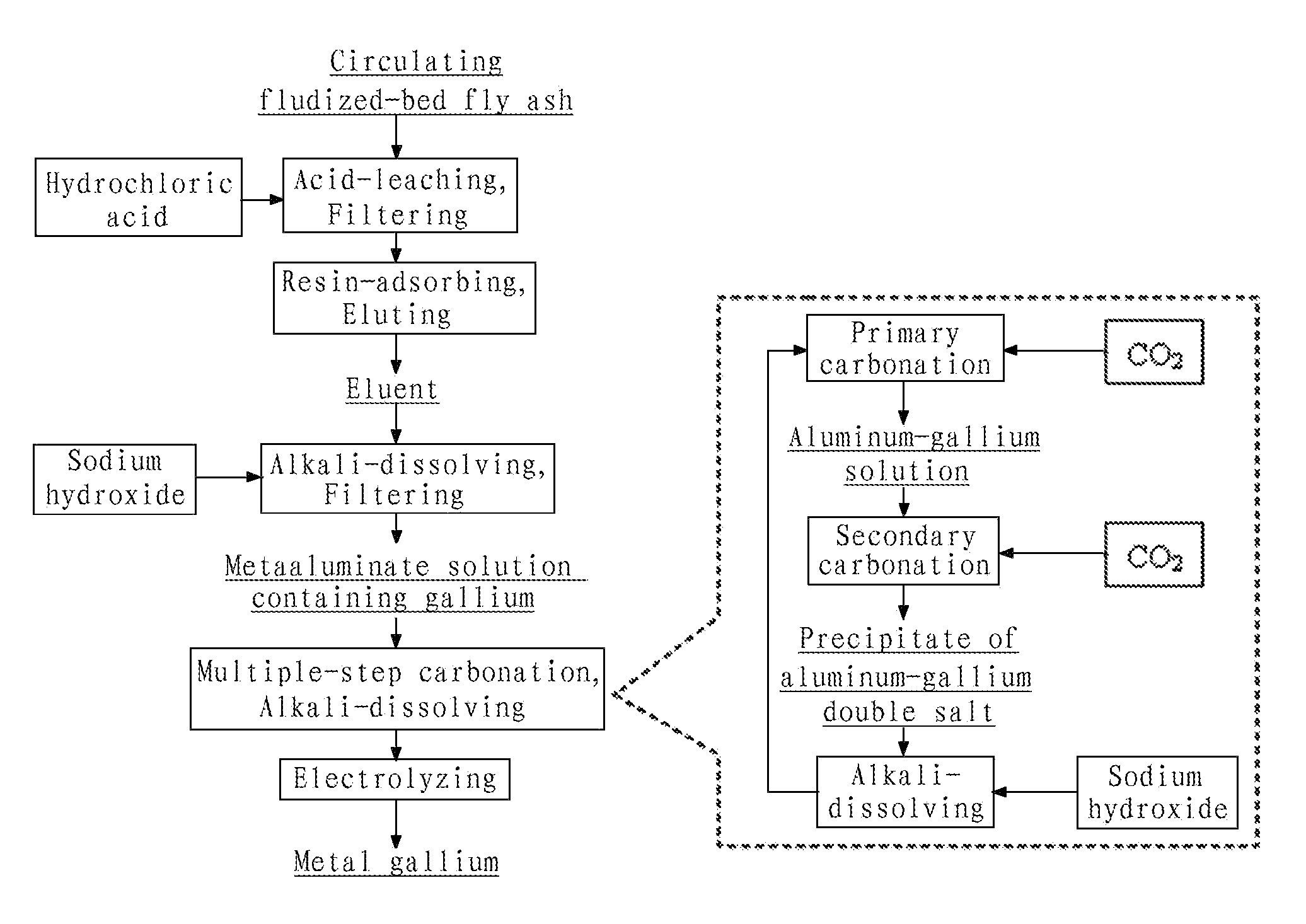
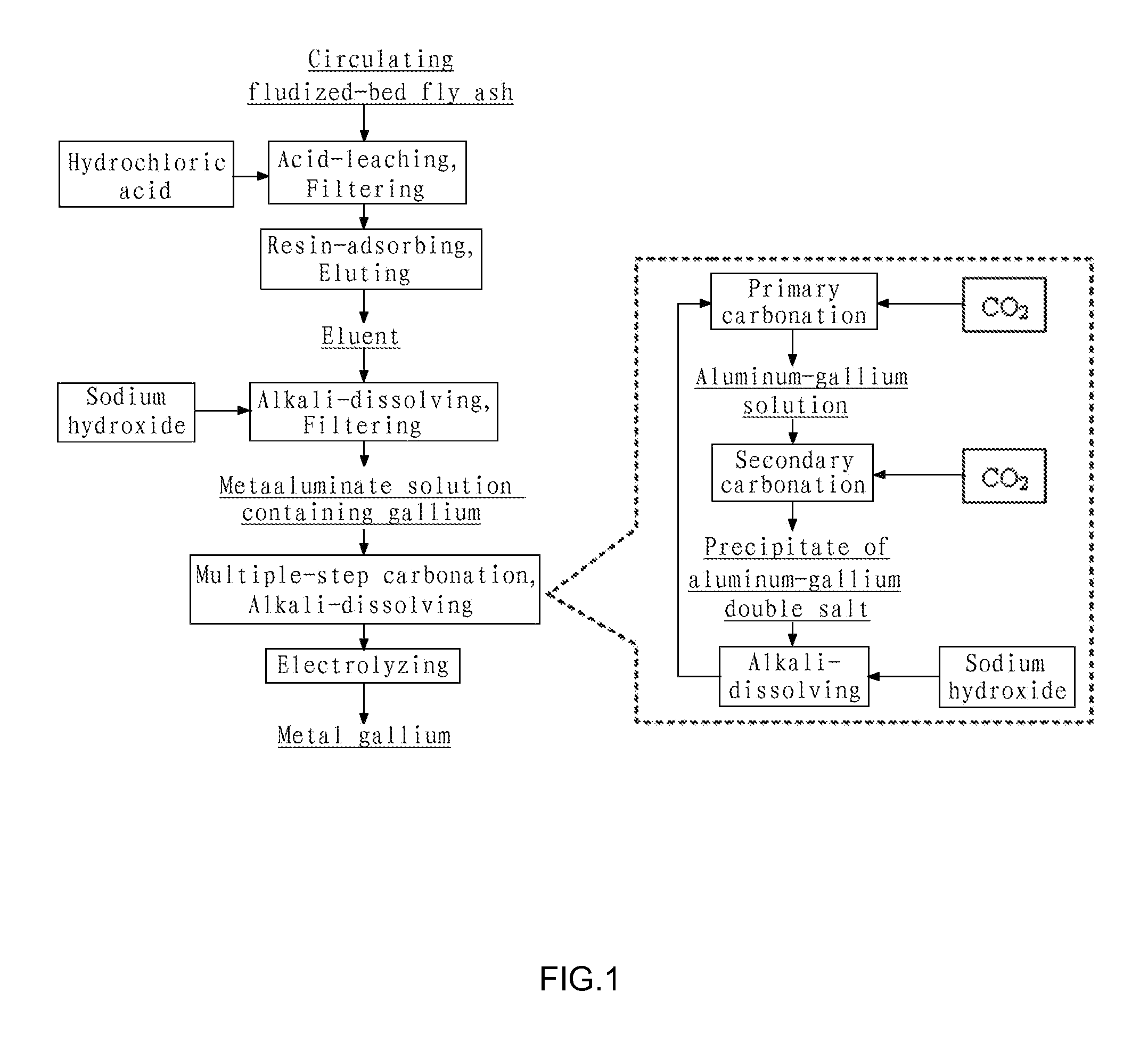
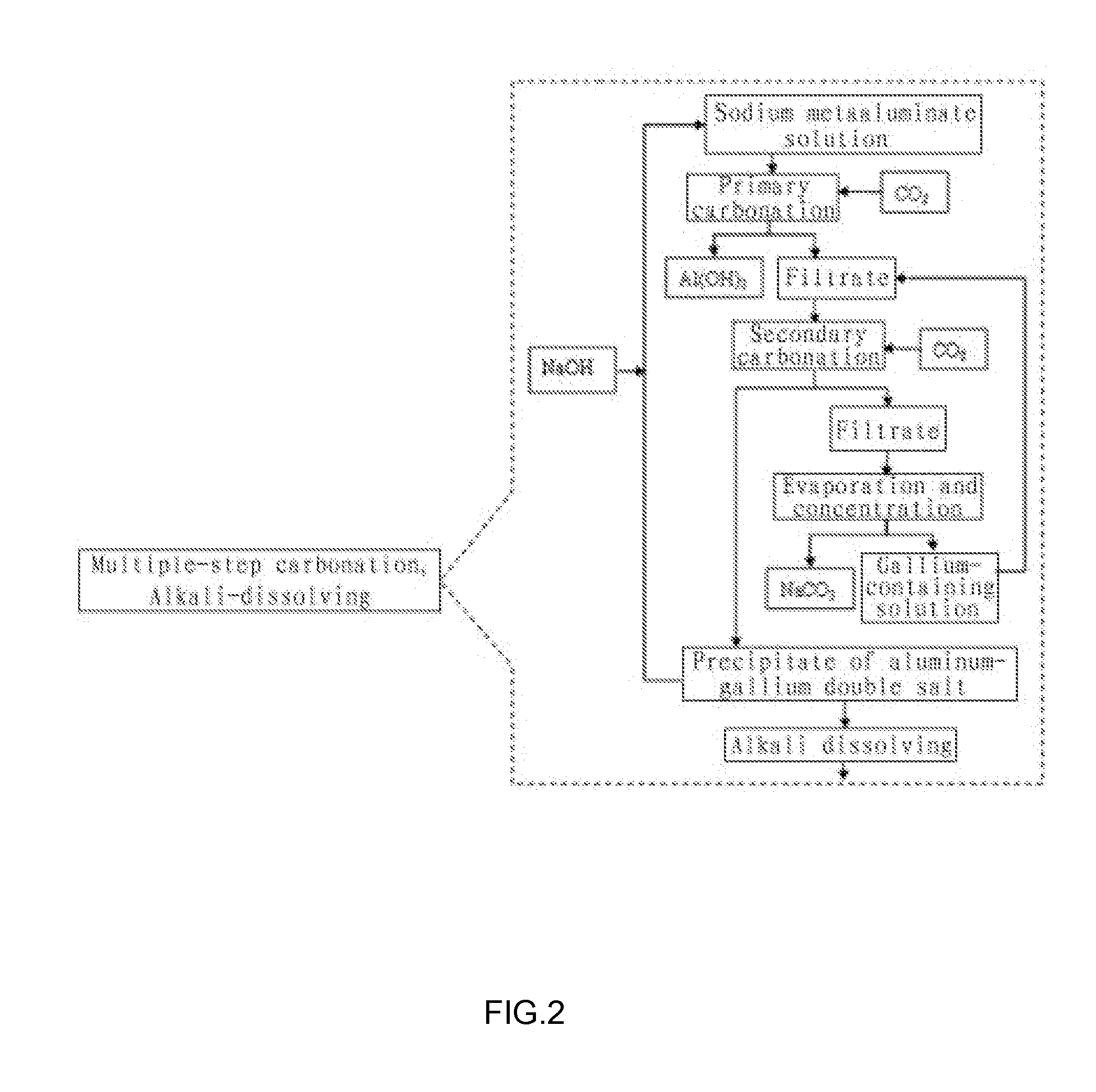
[0066](1) Crushing the circulating fluidized-bed fly ash to a size of 200 mesh, removing iron by wet magnetic separation using the vertical magnetic separator as illustrated in FIG. 3, such that the ferric oxide content in the fly ash was reduced to 0.8 wt %; putting the filtered cake of the fly ash obtained after magnetic separation into an acid-resistant reactor and adding industrial hydrochloride acid having a concentration of 37 wt % therein to perform acid dissolving reaction, wherein the molar ratio of HCl contained in the hydrochloride acid to alumina contained in the fly ash was 4.5:1, the reaction temperature was 200° C., the reaction pressure was 2.1 MPa and the reaction time was 1 hour; and then pressure-filtering the discharged reaction product by means of plate-and-frame filter press and washing to yield a hydrochloric leachate having pH of 1.7, wherein the leaching efficiency of gallium from ...
Example
Example 2
[0071]The operation conditions were the same as those of Example 1 except step (1). Step (1) was adjusted as follows:
[0072]Crushing the circulating fluidized-bed fly ash to a size of 150 mesh, removing iron by wet magnetic separation using the vertical magnetic separator as illustrated in FIG. 3, such that the ferric oxide content in the fly ash was reduced to 0.8 wt %; putting the filtered cake of the fly ash obtained after magnetic separation into an acid-resistant reactor and adding industrial hydrochloride acid having a concentration of 28 wt % therein to perform acid dissolving reaction, wherein the molar ratio of HCl contained in the hydrochloride acid to alumina contained in the fly ash was 5:1, the reaction temperature was 150° C., the reaction pressure was 1.0 MPa and the reaction time was 2 hours; and then pressure-filtering the discharged reaction product by means of plate-and-frame filter press and washing to yield a hydrochloric leachate having pH of 1.5, where...
Example
Example 3
[0074]The operation conditions were the same as those of Example 1 except step (1). Step (1) was adjusted as follows:
[0075]Crushing the circulating fluidized-bed fly ash to a size of 200 mesh, removing iron by wet magnetic separation using the vertical magnetic separator as illustrated in FIG. 3, such that the ferric oxide content in the fly ash was reduced to 0.8 wt %; putting the filtered cake of the fly ash obtained after magnetic separation into an acid-resistant reactor and adding industrial hydrochloride acid having a concentration of 20 wt % therein to perform acid dissolving reaction, wherein the molar ratio of HCl contained in the hydrochloride acid to alumina contained in the fly ash was 8:1, the reaction temperature was 100° C., the reaction pressure was 0.1 MPa and the reaction time was 4 h; and then pressure-filtering the discharged reaction product by means of plate-and-frame filter press and washing to yield a hydrochloric leachate having pH of 1.4, wherein t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap