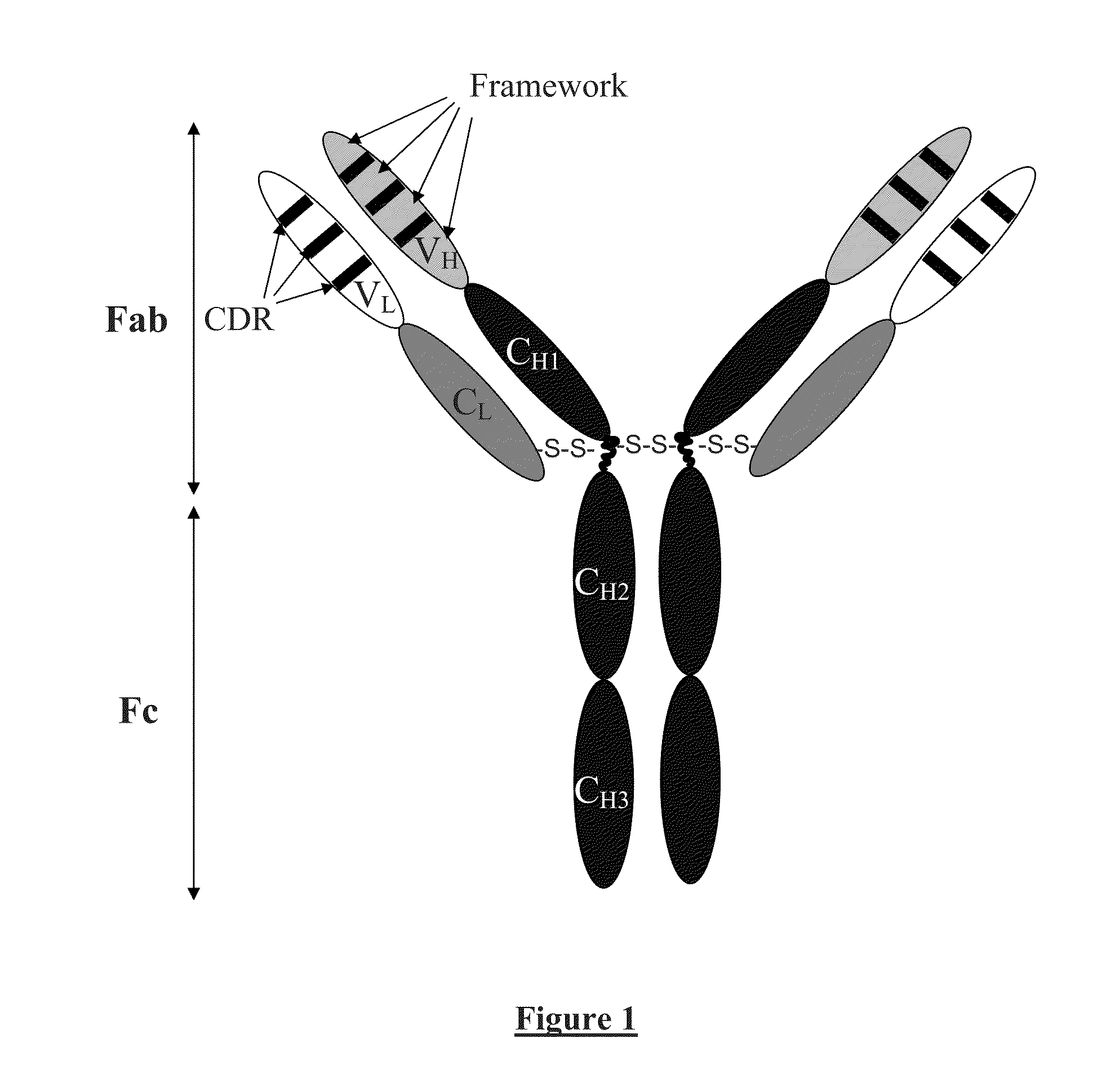
Chimeric Anti-ricin antibody
a technology of ricin and anti-ricin, which is applied in the direction of immunological disorders, drug compositions, peptides, etc., can solve the problems of ricin being extremely toxic, affecting the anti-ricin effect, so as to achieve the effect of conserving the resistance of anti-ricin properties and being easy to determin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction and Sequencing of an HK622-26 Expression Vector for the Expression of Antibodies According to the Invention
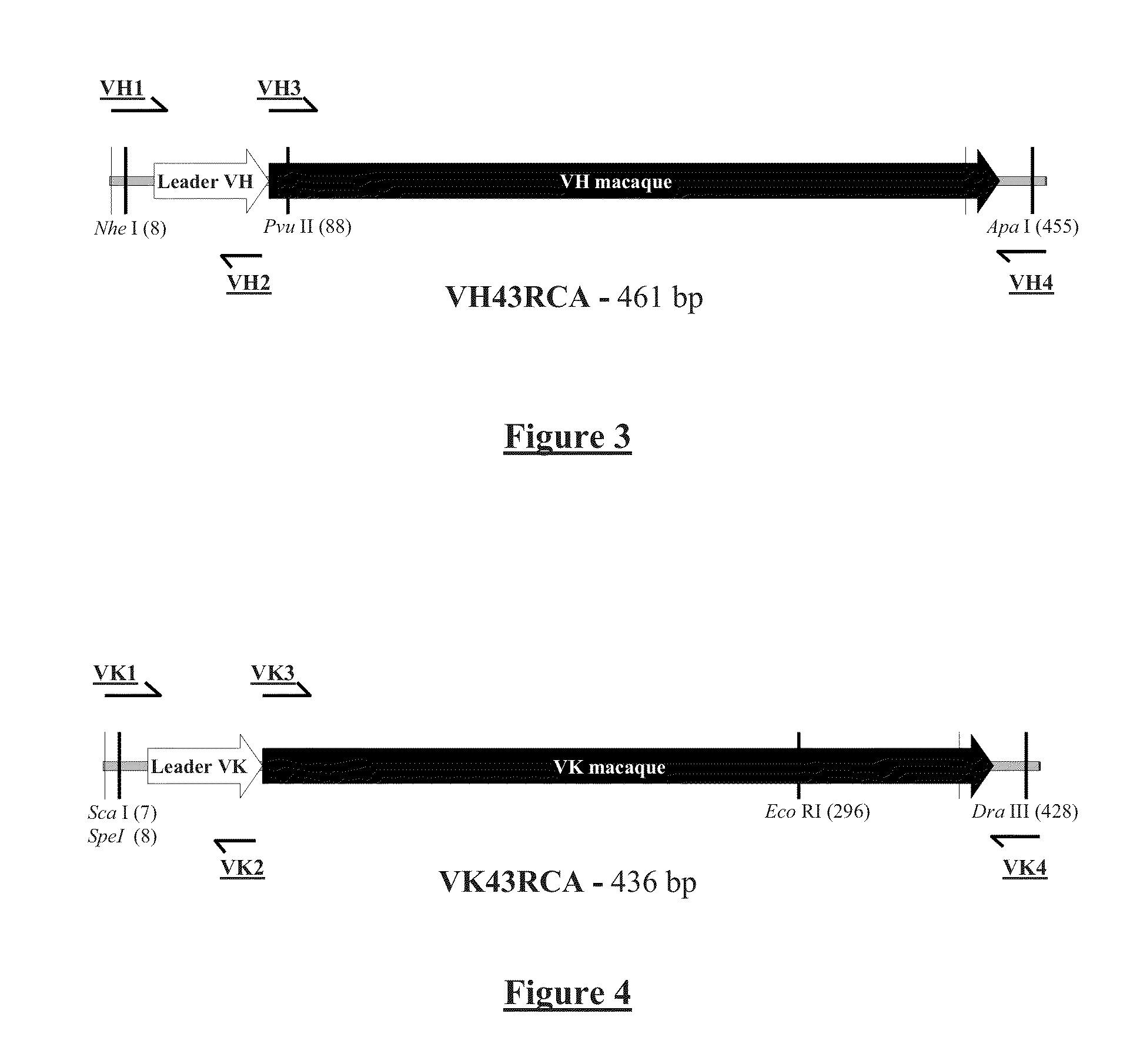
[0245]The expression vector HK622-26 (SEQ ID No 21) was constructed for the expression of the chimeric macaque-human (IgG) anti-ricin monoclonal antibody.
[0246]The HK622-26 vector was constructed from the CHK622-05 vector by ‘double chimerization,’ meaning the addition by PCR of assemblies from human leader regions, and by a cloning addition of the constant CK and CH human regions to the VH and VK macaque variable sequences.
[0247]The variable heavy and light chain VH and VK regions are extracted from a coding vector for an anti-ricin ScFv, ScFv43RCA / H2-V116, and are introduced into the ‘generic’ CHK622-08 vector, after adding leader sequences to ensure a good synthesis of the chimerical antibody. The CK and CH constant regions are of human origin, and are derived from clone T125-A2, directed against the Rhesus D antigen.
A—Synthesis of the VH43RCA Region by PCR Asse...
example 2
Obtaining Anti-Ricin-Producing Clones in the YB2 / 0 Line by Direct Double Transfection
[0321]This study has the aim of obtaining clones producing anti-ricin in the YB2 / 0 line by direct double transfection.
[0322]The YB2 / 0 cells were maintained (by re-treatment with 1×105 cell / ml twice per week) in light of transfection at 105 cells / ml in an EMS medium, 5% SVF.
[0323]The vectors utilised for the transfection are as follows: HK622-26 / EcoRV, H416-24 (T+) et K416-23 (T+). HK622-26 / EcoRV signifies that the vector obtained in example 1 was linearised by digestion with the Eco RV enzyme in order to promote its integration into the transformed cells.
Transfection
[0324]The cells are transfected according to the following protocol:
4 cuvettes containing 500 μL of cells are prepared in the following manner:[0325]Cuvettes 1 and 2: 42.8 μg of vector HK622-26 / EcoRV[0326]Positive control cuvette: 25.2 μg of vector H416-24, linearised;[0327]23.2 μg of vector H416-23, linearised;[0328]Negative control cuv...
example 3
Production of Antibodies in Roller Bottles According to the Invention
[0382]The EE9-5G7 clone was selected for the production of 500 mg of antibodies on the basis of the dosages of maximum static pseudo production which established its level of production as 19 mg / L and also based on the results of the stability study conducted on the parental EE9 cloid, whose production level was stable for six weeks.
[0383]On the basis of these data, clone EE9-5G7 was amplified in such a way as to achieve two successive productions of 30 roller bottles in batch mode. Production was stopped when the cell viability dropped below 50%. As the supernatant measured at the end of production was only 12 mg / L, namely 648 mg antibody produced. This supernatant was measured, centrifuged, filtered before being concentrated 15 times. The concentrate was purified by affinity chromatography and this allowed 468 mg purified antibody to be obtained.
[0384]The production of anti-ricin monoclonal antibody by clones IF2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com