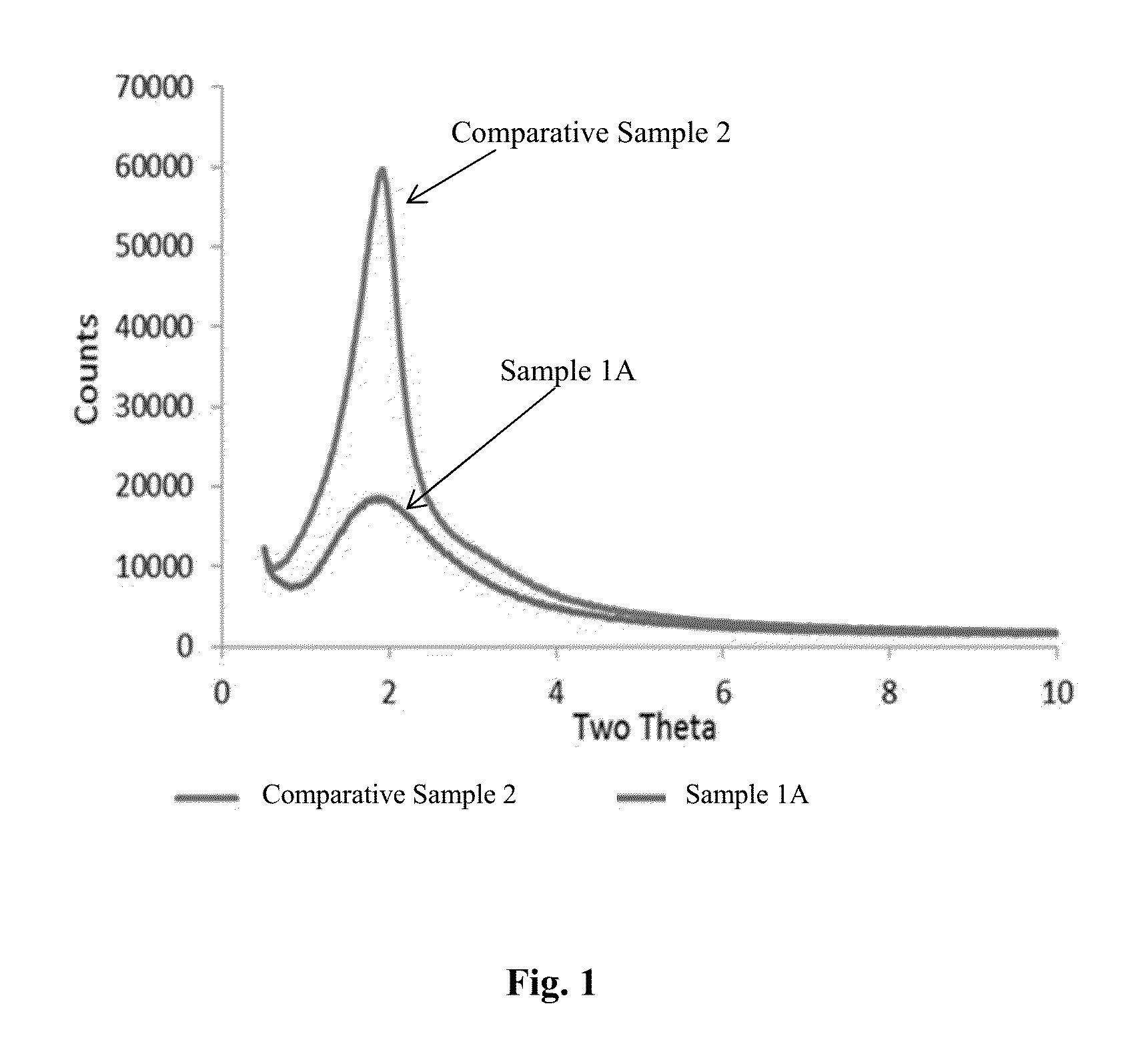
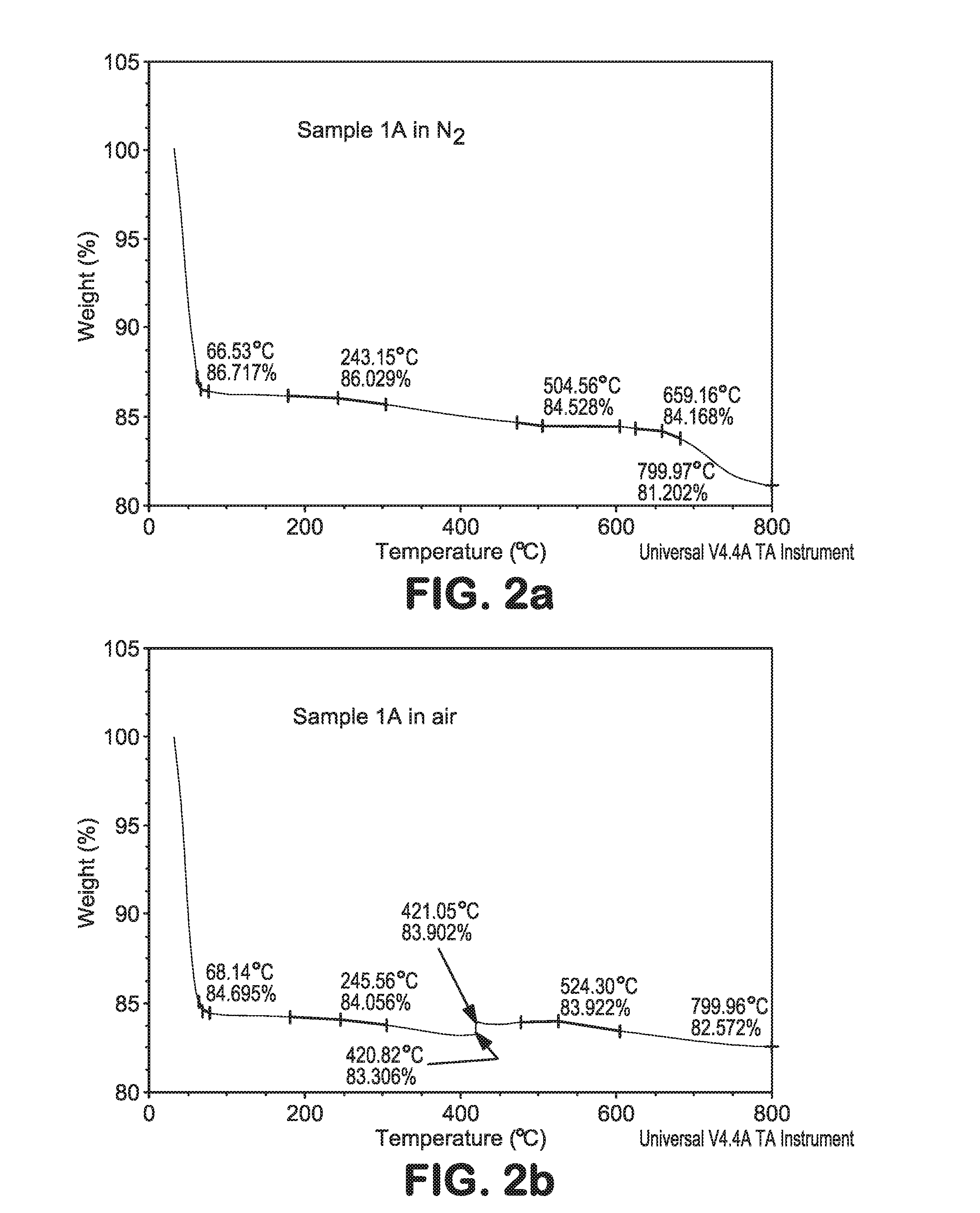
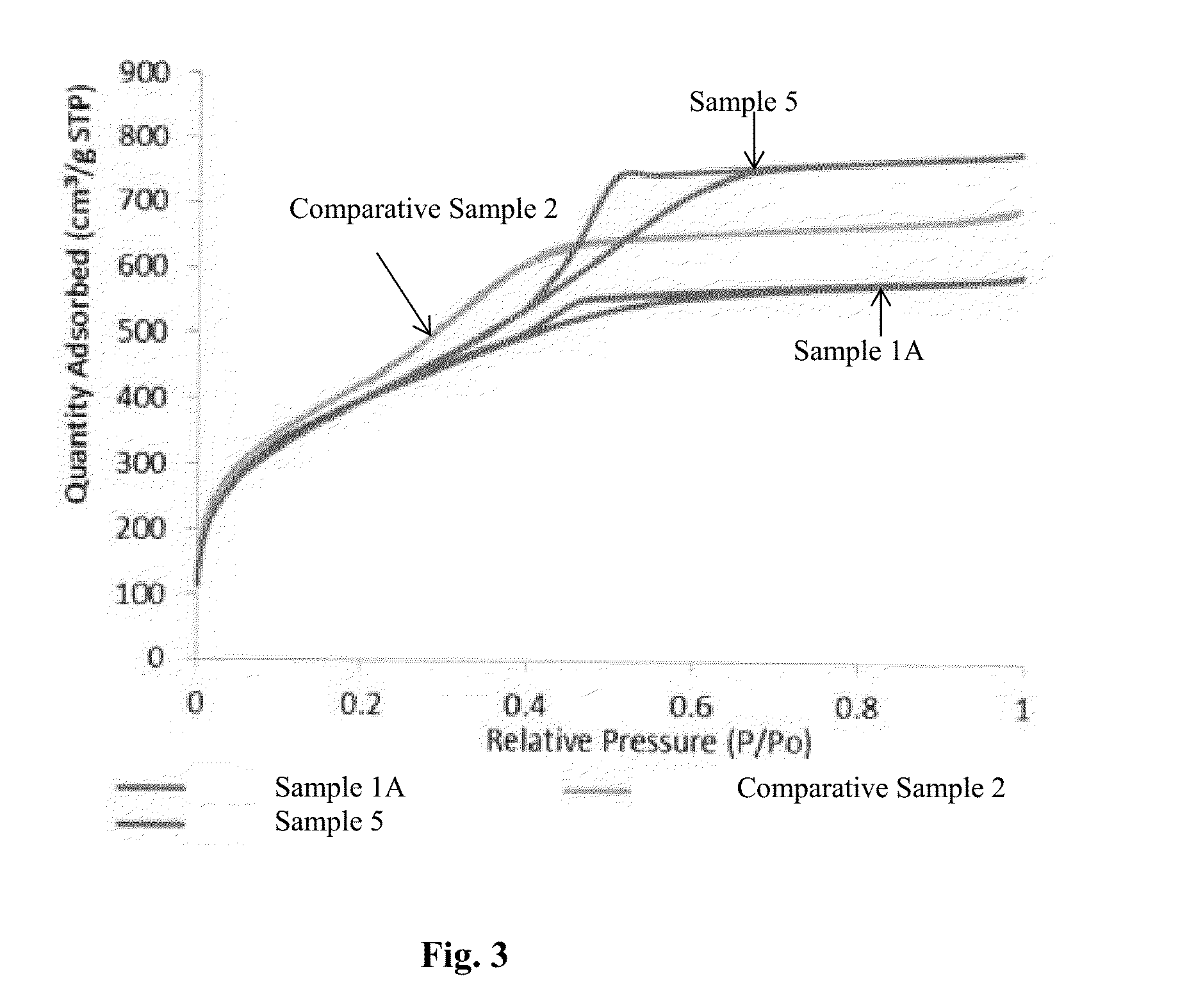
Organosilica materials and uses thereof
a technology of organic materials and materials, applied in the field of organic materials, can solve the problems of limiting the ability to scale-up the process for industrial applications, requiring a complicated process to eliminate, and reducing the application range of organic materials, etc., and achieve the effect of reducing the number of organic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0323]An organosilica material, which is a polymer of at least one independent monomer of Formula [Z1OZ2SiCH2]3 (I), wherein each Z1 represents a hydrogen atom, a C1-C4 alkyl group or a bond to a silicon atom of another monomer and each Z2 represents a hydroxyl group, a C1-C4 alkoxy group, a C1-C6 alkyl group or an oxygen atom bonded to a silicon atom of another monomer and at least one other monomer selected from the group consisting of:[0324](a) an independent unit of formula Z3OZ4Z5Z6Si (II), wherein each Z3 represents a hydrogen atom or a C1-C4 alkyl group or a bond to a silicon atom of another monomer; and Z4, Z5 and Z6 are each independently selected from the group consisting of a hydroxyl group, a C1-C4 alkyl group, a C1-C4 alkoxy group, and an oxygen atom bonded to a silicon atom of another monomer;[0325](b) an independent unit of formula Z7Z8Z9Si—R—SiZ7Z8Z9 (III), wherein each Z7 independently represents a hydroxyl group, a C1-C4 alkoxy group or an oxygen atom bonded to a s...
embodiment 2
[0327]The organosilica material of embodiment 1, wherein each Z1 represent a hydrogen atom, a C1-C2 alkyl group or a bond to a silicon atom of another monomer and each Z2 represents a hydroxyl group, a C1-C4 alkyl group, a C1-C2 alkoxy group or an oxygen atom bonded to a silicon atom of another monomer.
embodiment 3
[0328]The organosilica material of embodiment 1 or 2, wherein each Z1 represent a hydrogen atom, ethyl or a bond to a silicon atom of another monomer and each Z2 represents a hydroxyl group, methyl, ethoxy or an oxygen atom bonded to a silicon atom of another monomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore diameter | aaaaa | aaaaa |
| pore diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com