High-concentration monoclonal antibody formulations
a monoclonal antibody, high-concentration technology, applied in the direction of antibody medical ingredients, immunological disorders, pharmaceutical active ingredients, etc., can solve the problems of concentration-dependent solution viscosity, formulation scientists are constantly challenged, and restrict product formulations, etc., to achieve acceptable injectability, low viscosity, and mature manufacturing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
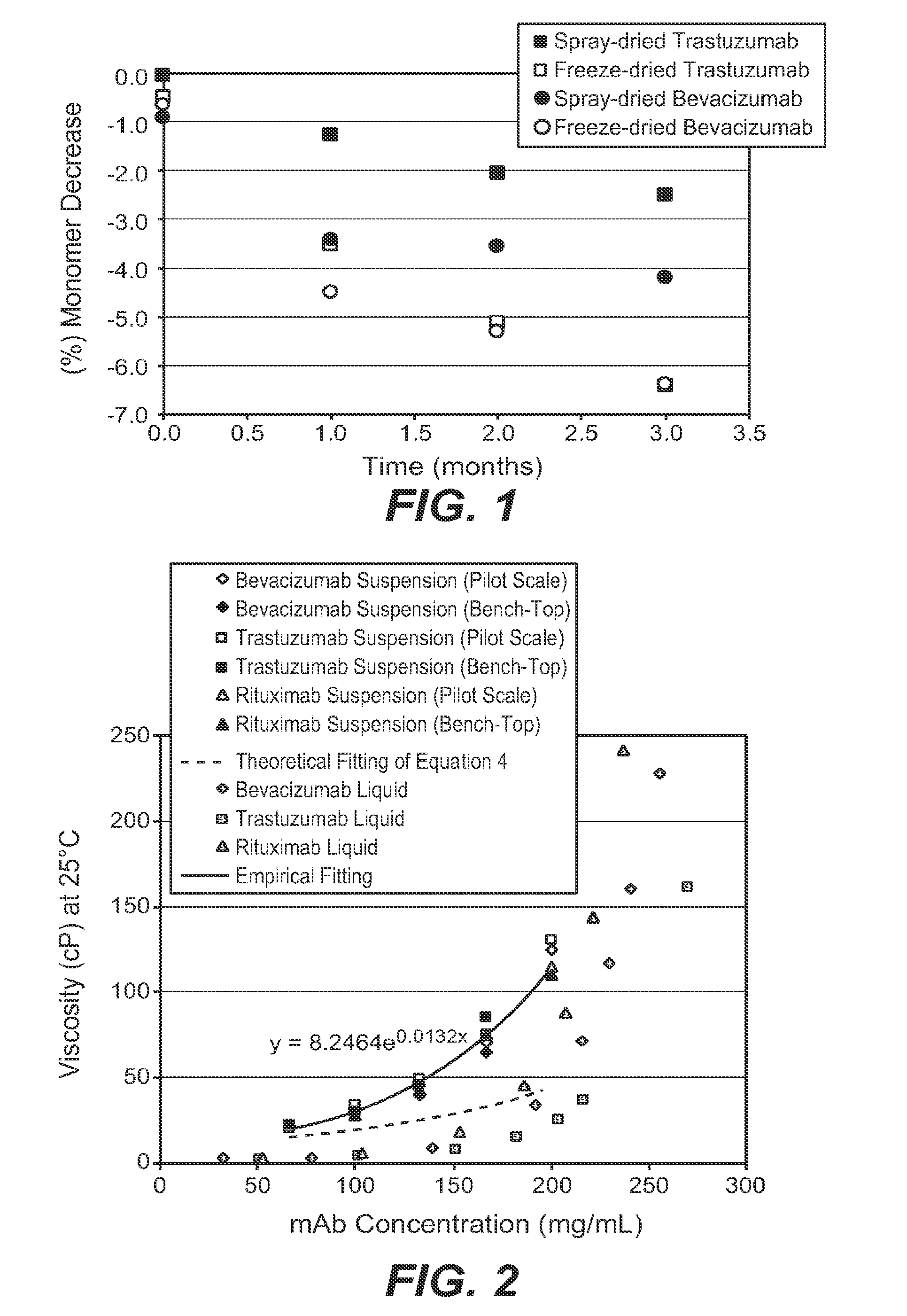
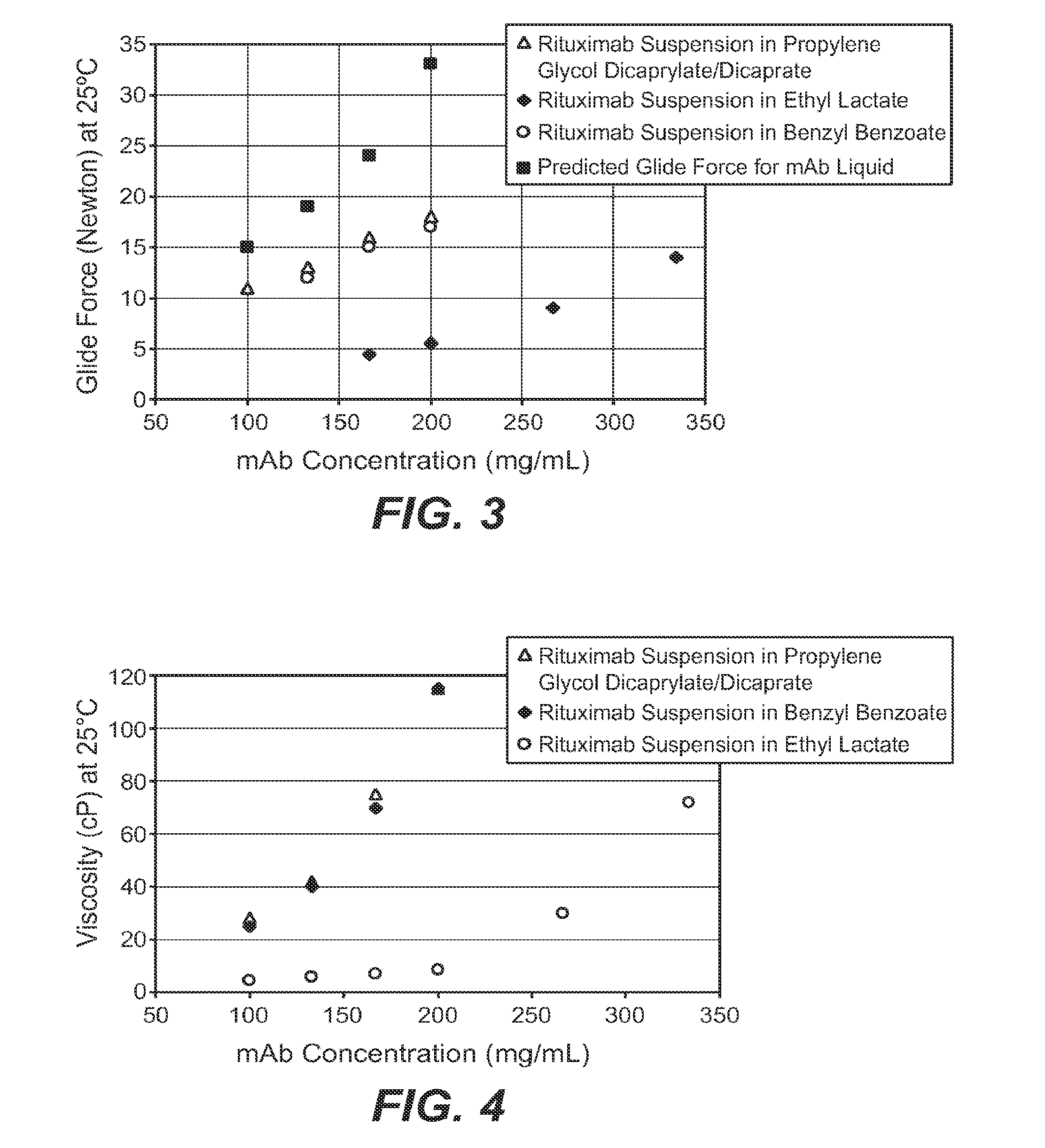
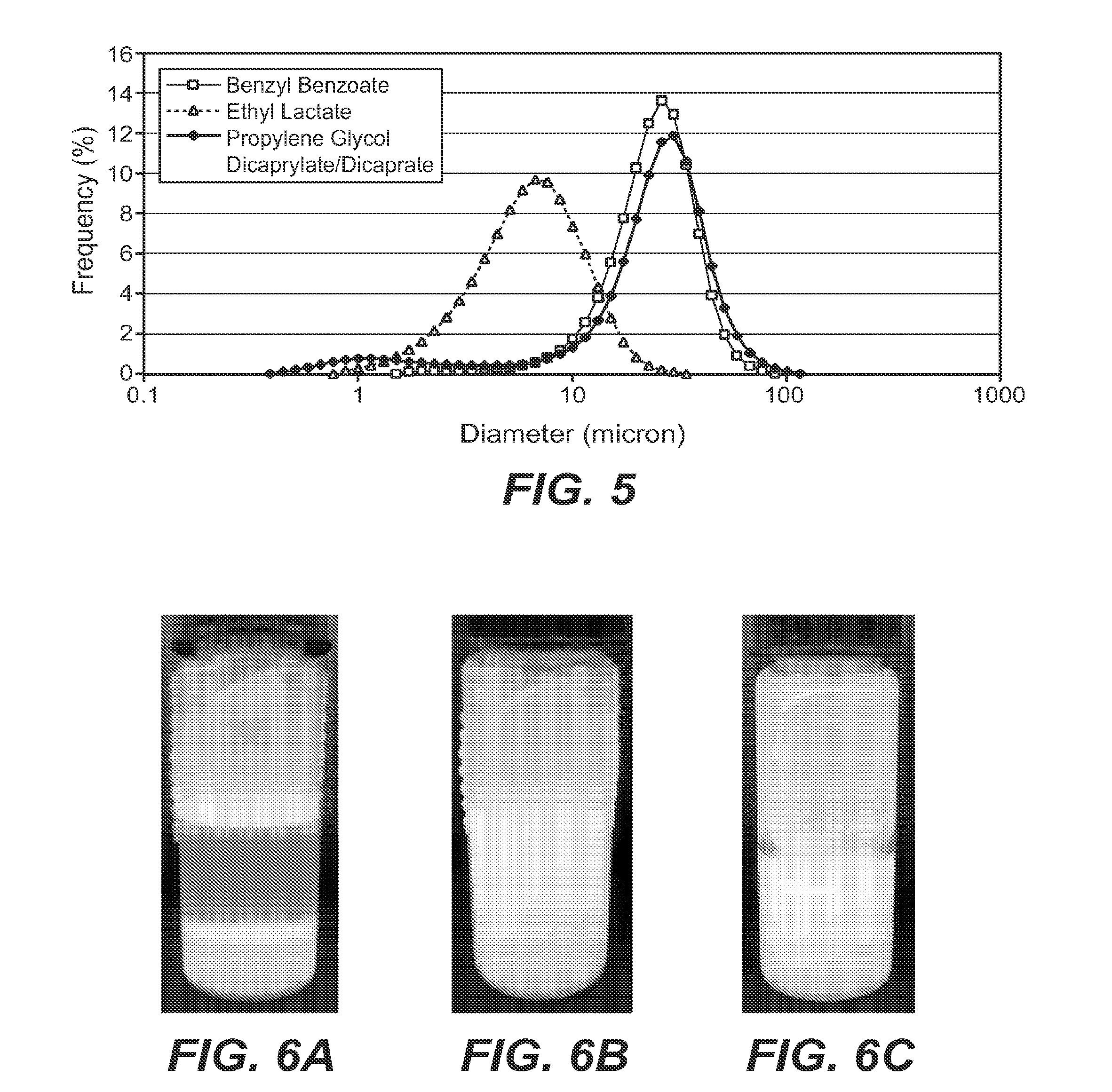
[0168]Developing high-concentration monoclonal antibody liquid formulations (≧200 mg / mL) for subcutaneous (SC) administration is often challenging with increased viscosity that makes injection difficult. This investigation was intended to overcome this obstacle using a non-aqueous powder suspension approach. Three human IgG1 monoclonal antibodies were spray dried and suspended in a suspension vehicle at different monoclonal antibody concentrations. Propylene glycol dicaprylate / dicaprate, benzyl benzoate, and ethyl lactate were employed as model suspension vehicles. Suspensions were characterized for viscosity, particle size, and syringeability. Physical stability of the suspension was visually inspected. The suspensions in general outperformed the liquid solutions in terms of injectability despite higher viscosity at the same monoclonal antibody concentrations. Powder formulations and powder properties appeared to have little effect on suspension viscosity or injectability. Among th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com