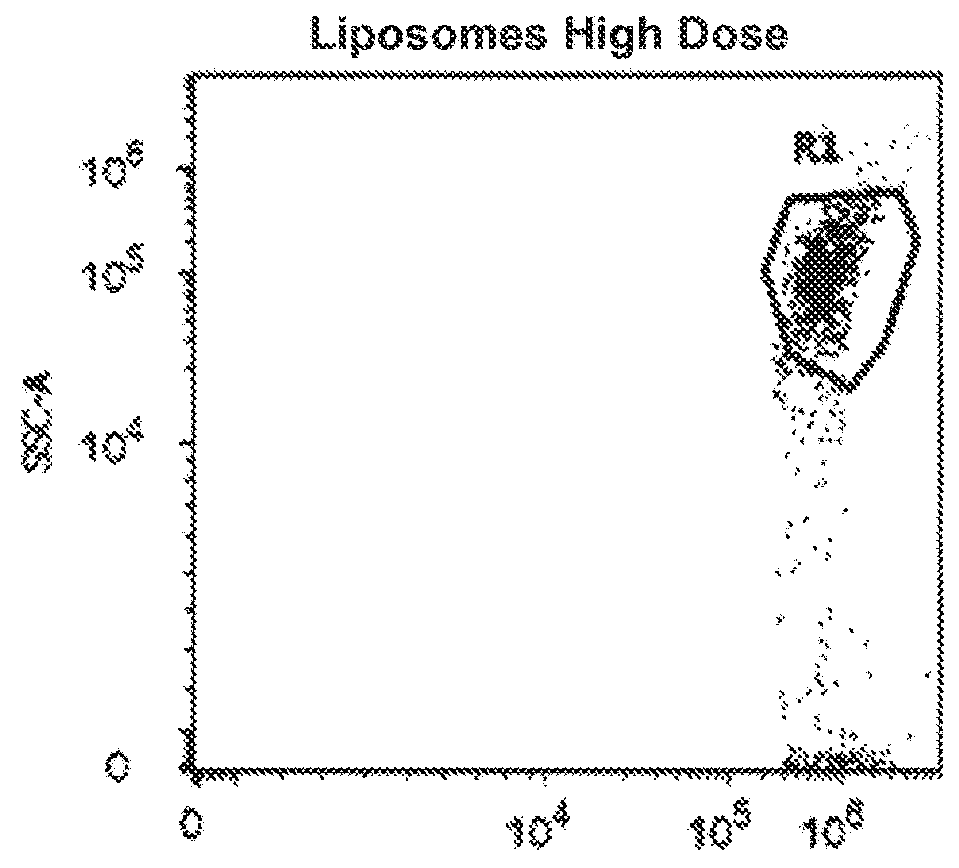
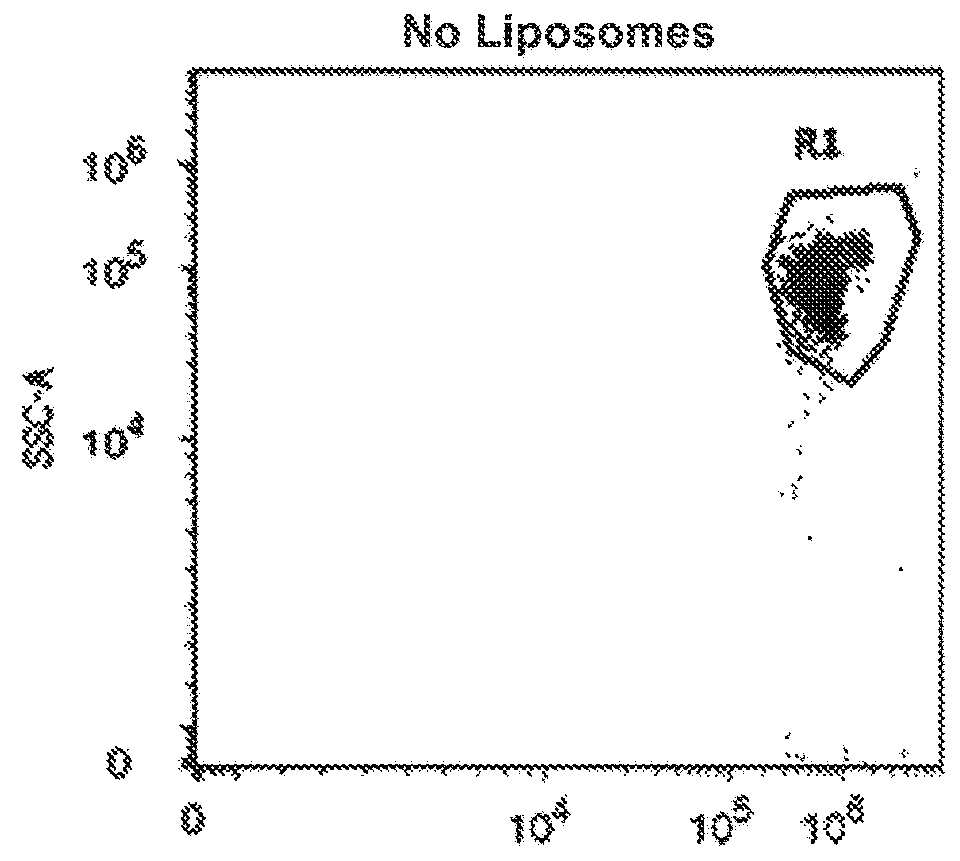
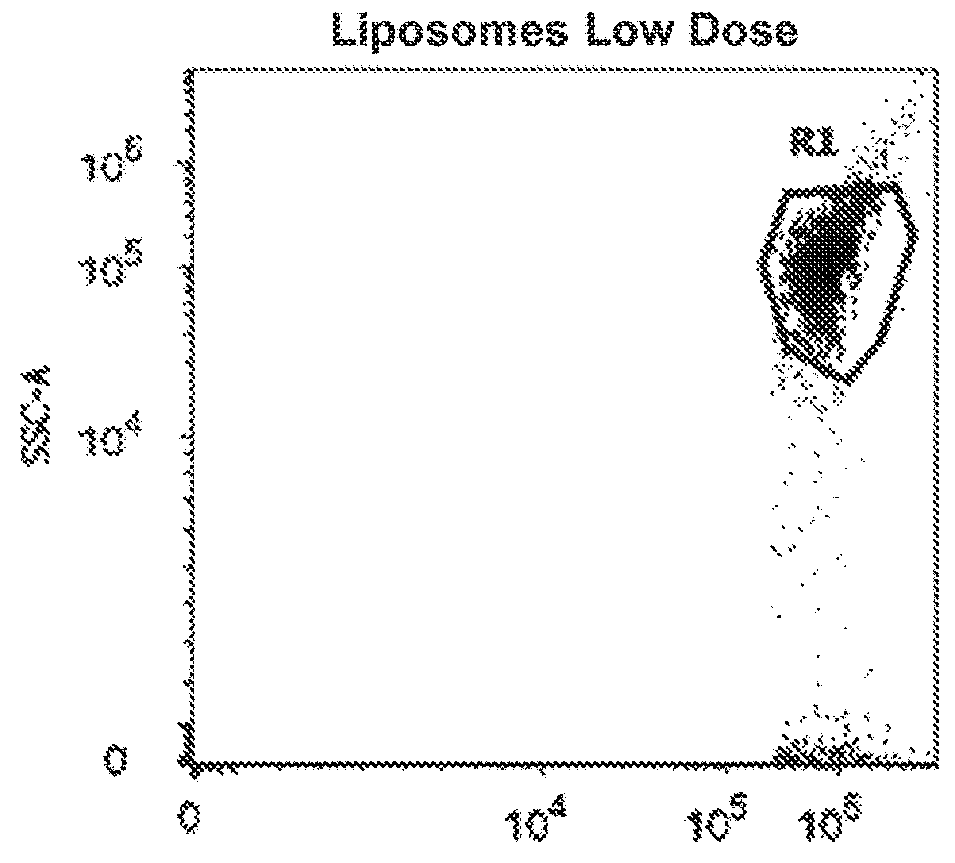
Synthetic membrane-receiver complexes
a membrane receptor and complex technology, applied in the field of pharmaceutical compositions, can solve the problems of limited therapeutic effect of compositions that alleviate or prevent diseases and conditions associated with the circulatory system, limited therapeutic activity, and limited potential therapeutic applications of technologies based on erythrocyte ghosts, so as to reduce the circulatory concentration of the target metabolite and effectively increase the circulatory concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
mbly
[1264]DNA encoding the following genes—glycophorin A (Uniprot ID P02724), Kell (Uniprot ID P23276), antibody scFv against hepatitis B surface antigen (Bose et al. 2003 Mol Immunol 40(9):617, GenBank ID AJ549501.1), adenosine deaminase (Uniprot ID P00813), phenylalanine hydroxylase from Chromobacterium violaceum (GenBank ID AF146711.1), complement receptor 1 (Uniprot ID P17927), CD46 (GenBank: BAA12224.1), CD55 (Uniprot ID P08174), CD59 (Uniprot ID P13987), green fluorescent protein (Uniprot ID P42212), thymidine phosphorylase (Uniprot ID P19971), glucocerebrosidase (Uniprot ID P04062), beta2 glycoprotein 1 (Uniprot ID P02749), phospholipase a2 receptor (Uniprot ID Q13018), collagen alpha-3(IV) (Uniprot ID Q01955), serum amyloid P (Uniprot ID P02743), lipoprotein lipase (Uniprot ID P06858), asparaginase (Uniprot ID P00805), factor IX (Uniprot ID F2RM35), ADAMTS13 (Uniprot ID Q76LX8)—were purchased as cDNA from Dharmacon (GE Life Sciences) or synthesized de novo by DNA2.0 and Gens...
example 2
mbly
[1275]A gene of interest is cloned into the multiple cloning site of the pSP64 vector (Promega) using standard molecular biology methods. The vector is digested with EcoRI (NEB) to generate a linearized dsDNA vector containing the SP6 promoter, gene of interest, and 30 nucleotide long poly-A tail. mRNA is synthesized by reaction with SP6 RNA polymerase (Promega) according to manufacturer's instructions, including recommended concentrations of 5′ cap analog (ARCA) to synthesize capped mRNA transcript. The reaction mixture is then treated with DNAse to digest the template vector (Riboprobe from Promega) and the mRNA is purified using the EZNA MicroElute RNA Clean-Up kit (Omega).
example 3
ure
[1276]1. Human Red Blood Cells (RBCs)
[1277]CD34 cells are isolated from peripheral blood by supermagnetic microbead selection by the use of Mini-MACS columns (Miltenyi Biotec; 94%+ / −3% purity). The cells are cultured in erythroid differentiation medium (EDM) on the basis of IMDM supplemented with stabilized glutamine, 330 μg / mL holo-human transferrin, 10 μg / mL recombinant human insulin, 2 IU / mL heparin, and 5% solvent / detergent virus-inactivated plasma. The expansion procedure comprises 3 steps. In the first step (day 0 to day 7), 10̂4 / mL CD34+ cells are cultured in EDM in the presence of 1 μM hydrocortisone, 100 ng / mL SCF, 5 ng / mL IL-3, and 3 IU / mL EPO. On day 4, 1 volume of cell culture is diluted in 4 volumes of fresh medium containing SCF, IL-3, EPO, and hydrocortisone. In the second step (day 7 to day 11), the cells are resuspended at 10̂5 / mL in EDM supplemented with SCF and EPO. In the third step (day 11 to day 18), the cells are cultured in EDM supplemented with EPO alone....
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid sequence | aaaaa | aaaaa |
| osmotic membrane fragility | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com