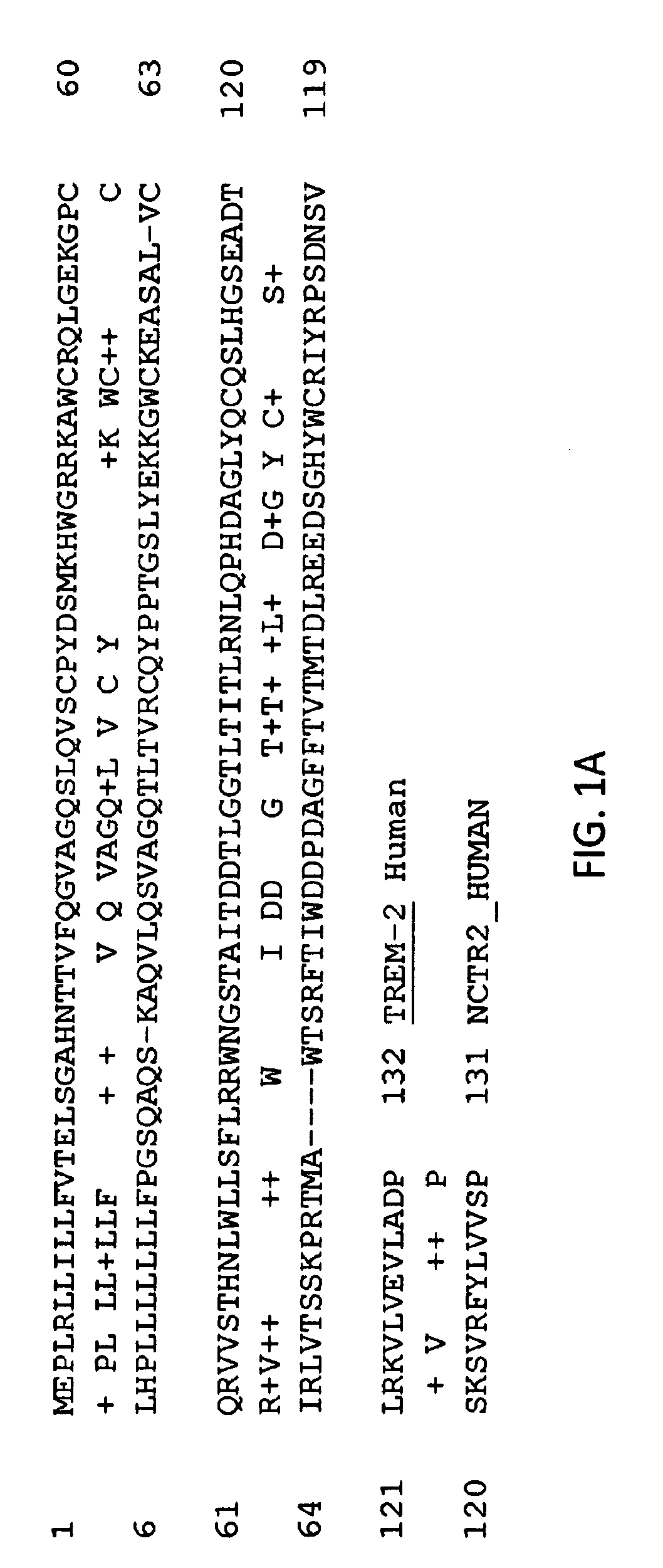
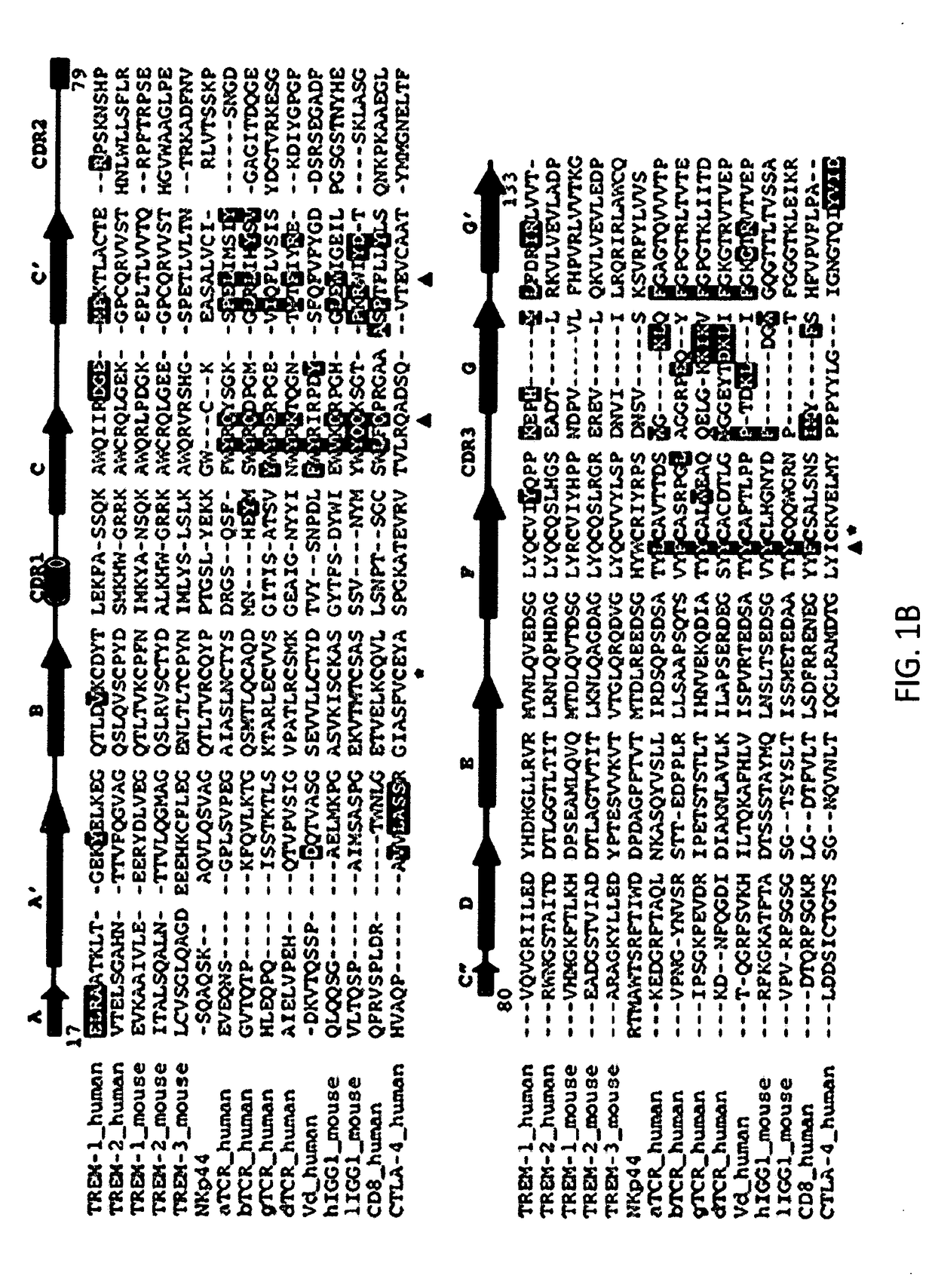
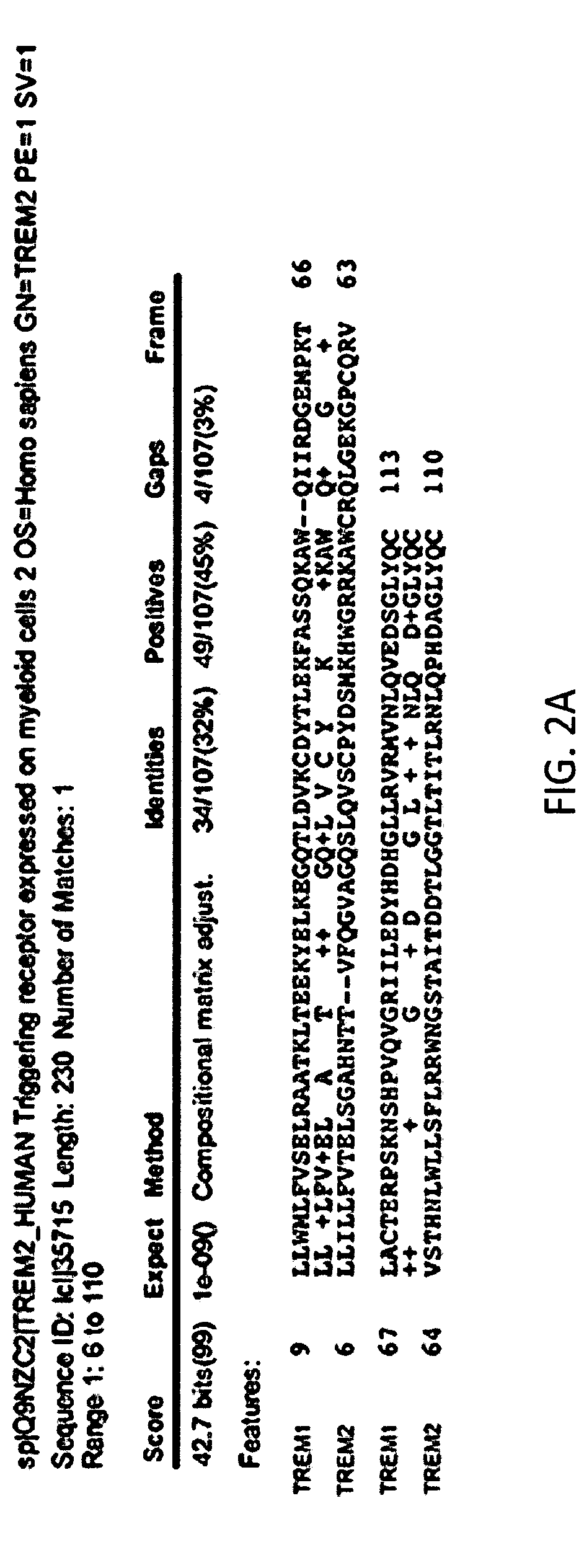
Anti-trem2 antibodies and methods of use thereof
a technology of anti-trem2 and antibodies, which is applied in the field of anti-trem2 and anti-dap12 antibodies, can solve the problems of a 3 fold increase in the risk of developing alzheimer's disease, the loss of function of trem2, and the effect of carrying these mutations is just as serious, so as to induce antigen-specific t-cell proliferation, reduce the expression of tnf-, and increase the expression of c-
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
2. The isolated antibody of embodiment 1, wherein the TREM2 protein, the DAP12 protein, or both is a mammalian protein or a human protein.
embodiment 2
3. The isolated antibody of embodiment 2, wherein the TREM2 protein, the DAP12 protein, or both is a wild-type protein.
4. The isolated antibody of embodiment 2, wherein the TREM2 protein, the DAP12 protein, or both is a naturally occurring variant.
5. The isolated antibody of any one of embodiments 1-4, wherein the one or more TREM2 activities comprise TREM2 binding to DAP12.
6. The isolated antibody of any one of embodiments 1-4, wherein the one or more DAP12 activities comprise DAP12 binding to TREM2.
7. The isolated antibody of any one of embodiments 1-6, wherein the one or more TREM2 activities, DAP12 activities, or both comprise DAP12 phosphorylation.
embodiment 7
8. The isolated antibody of embodiment 7, wherein DAP12 phosphorylation is induced by one or more SRC family tyrosine kinases.
9. The isolated antibody of any one of embodiments 1-8, wherein the one or more TREM2 activities, DAP12 activities, or both comprise PI3K activation.
10. The isolated antibody of any one of embodiments 1-9, wherein the one or more TREM2 activities, DAP12 activities, or both comprise increased expression of one or more anti-inflammatory mediators selected from the group consisting of IL-12p70, IL-6, and IL-10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com