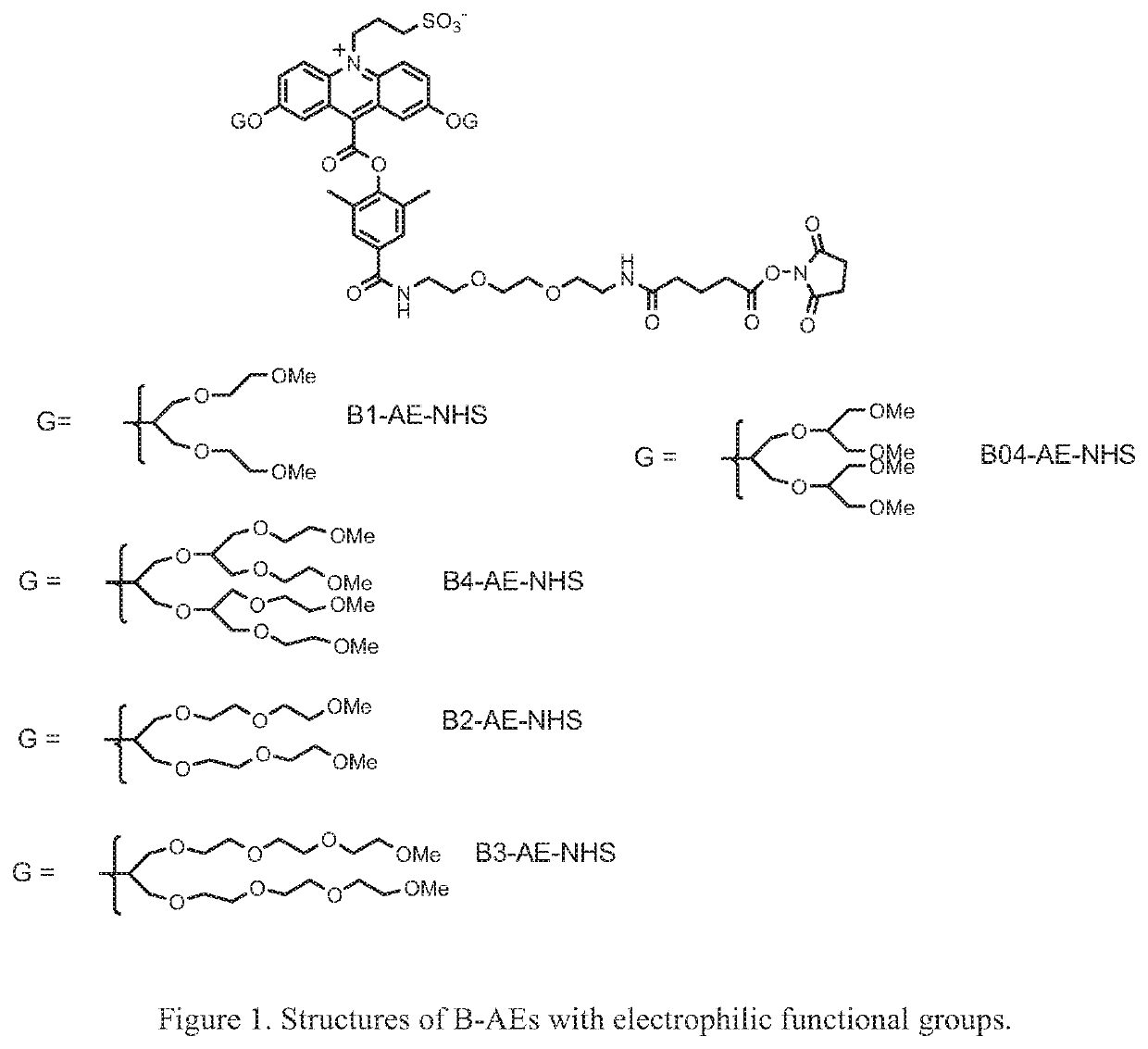
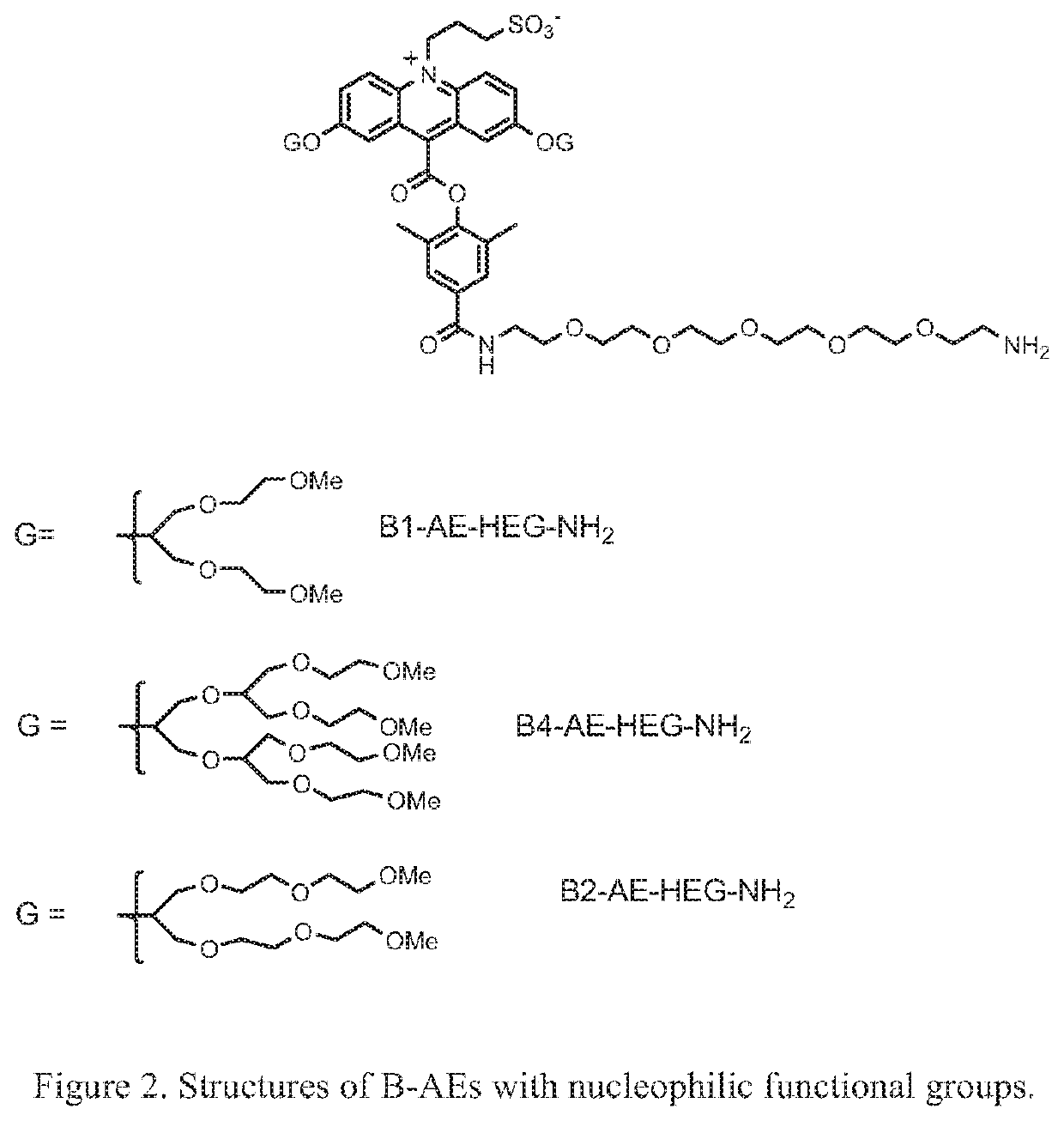
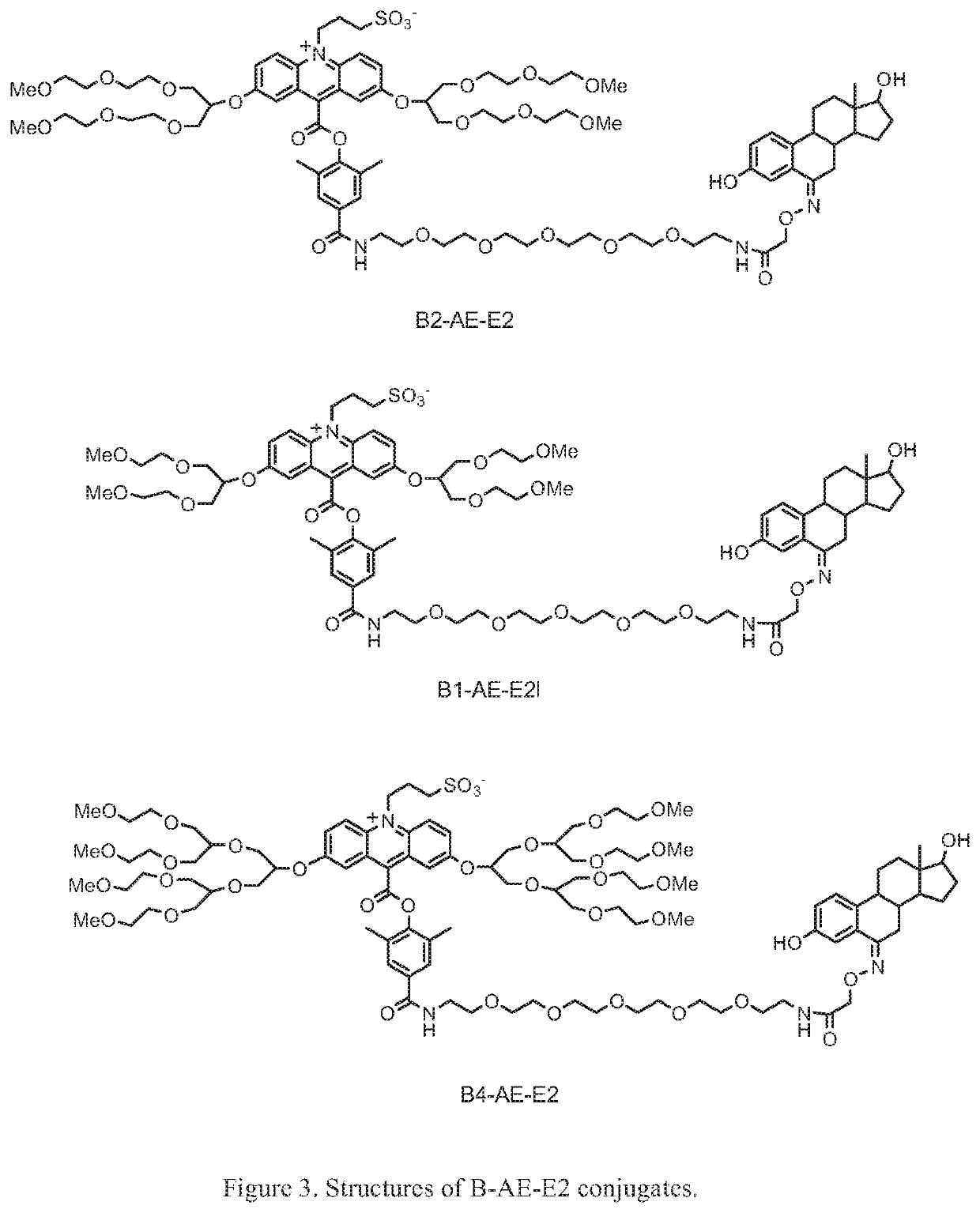
Hydrophilic high quantum yield acridinium esters with improved stability and fast light emission
a technology of acridinium esters and acridinium esters, which is applied in the field of hydrophilic, high quantum yield, chemiluminescent acridinium compounds, can solve the problems of limiting commercial utility, and achieve the effects of improving stability, high quantum yield and increasing light outpu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of B1-AE-NHS ester, 1i
a) 1,3-Bis(methoxyethoxy)-2-propyl toluenesulfonate, 1b
[0106]The compound 1a, 1,3-bis(methoxyethoxy)-2-propanol was synthesized as described by Cormier and Gregg in Chem. Mater. 1998, 10, 1309-1319. A solution of 1a (2 g, 9.6 mmol) in anhydrous pyridine (10 mL) was treated with 4-dimethylaminopyridine (0.234 g, 1.92 mmol) followed by p-toluenesulfonyl chloride (3.67 g, 19.25 mmol). The reaction was stirred at room temperature under a nitrogen atmosphere for 3 days. The solvent was then removed under reduced pressure and the residue was partitioned between ethyl acetate (75 mL) and 10% HCl (100 mL). The ethyl acetate layer was washed with brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product (4.05 g) was purified by flash chromatography on silica gel using 1:1, ethyl acetate / hexanes as eluent. The product was recovered as a light yellow oil. Yield=2.42 g (70%).
b) Compound 1d
[0107]A mixture of 2,7-dihyd...
example 2
Synthesis of B2-AE-NHS ester, 2h
a). 1,3-Bis(3,6-dioxaheptanyl)glycerol-2-toluenesulfonate, 2b
[0112]The compound 1,3-Bis(3,6-dioxaheptanyl)glycerol, 2a, was synthesized as described by Vacus and Simon in Adv. Mater. 1995, 7, 797-800. Crude 2a (16 g, 0.054 mol) was dissolved in anhydrous pyridine (50 mL) and treated with 4-dimethylaminopyridine (1.32 g, 0.011 mol) followed by p-toluenesulfonyl chloride (12.4 g, 0.065 mol). The reaction was stirred under a nitrogen atmosphere for 16 hours. The solvent was then removed under reduced pressure and the residue was partitioned between ethyl acetate (100 mL) and 2N HCl (100 mL). The ethyl acetate layer was washed with saturated sodium bicarbonate solution followed by brine. It was then dried over magnesium sulfate and concentrated under reduced pressure. The crude product (14 g) was purified by flash chromatography on silica gel using 1:4, hexanes / ethyl acetate as eluent. Yield=6.3 g, light yellow oil. MALDI-TOF MS 473.4 observed, (M+Na+).
b)...
example 3
B3-AE-NHS ester 3h
a) 1,3-Bis(3,6,9-dioxadecanyl)glycerol-2-toluenesulfonate, 3b
[0119]The compound 1,3-Bis(3,6,9-dioxadecanyl)glycerol, 3a, was synthesized as described by Lauter et al. in Macromol. Chem. Phys. 1998, 199, 2129-2140. The alcohol (7 g, 0.0182 mol) was dissolved in anhydrous pyridine (30 mL) and treated with 4-dimethylaminopyridine (0.444 g, 3.6 mmol) and p-toluenesulfonyl chloride (3.85 g, 0.02 mol). The reaction was stirred under a nitrogen atmosphere for 3 days. The solvent was then removed under reduced pressure and the residue was partitioned between ethyl acetate (100 mL) and 10% HCl (100 mL). The ethyl acetate layer was washed with saturated sodium bicarbonate solution and brine. It was then dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by flash chromatography on silica gel using 5:4.5:0.5, hexanes:ethyl acetate:methanol. Yield=4.47 g (45%); light yellow oil.
b) Compound 3c
[0120]A mixture of 1c (0.2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| storage temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap