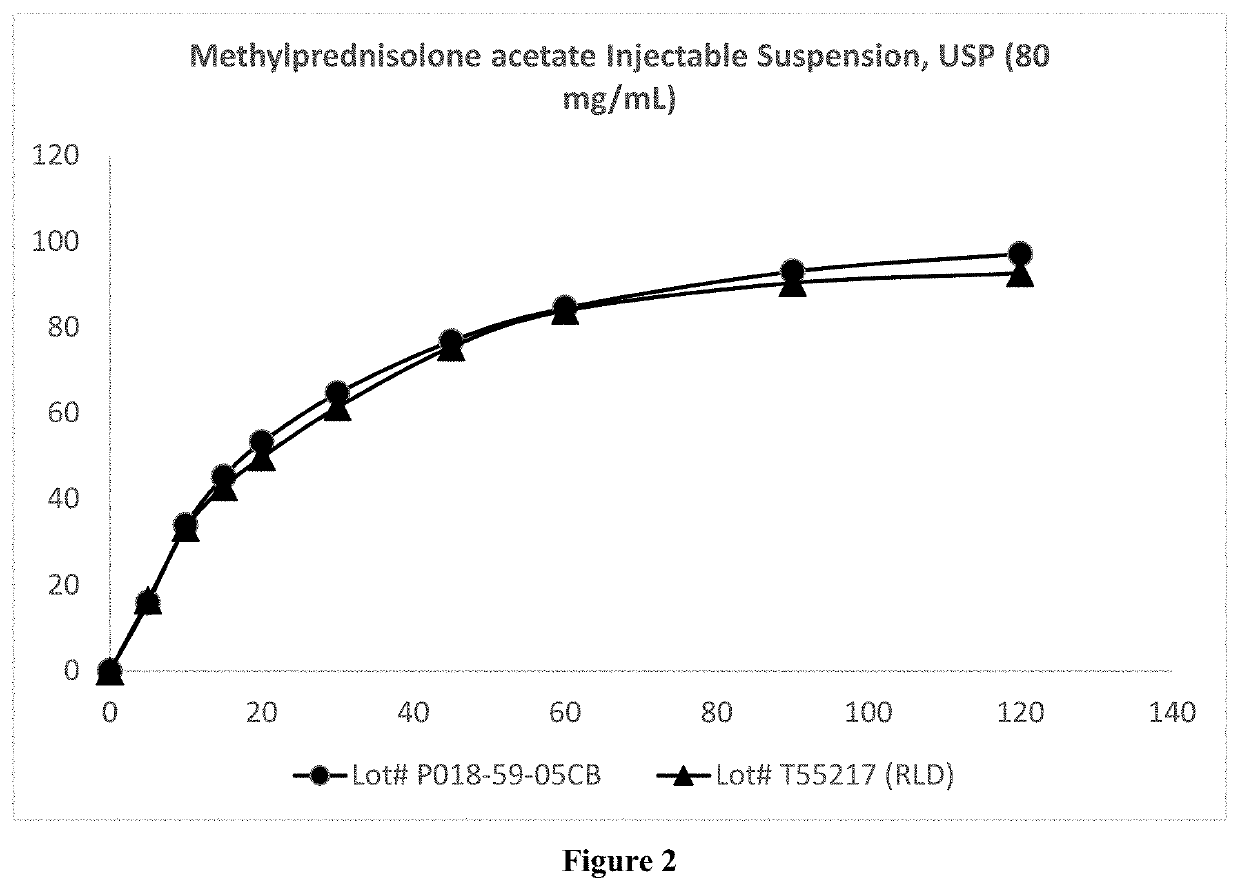
Preparation of Microparticulate Methylprednisolone Acetate Suspension
a technology of methylprednisolone acetate and microparticulate, which is applied in the direction of emulsion delivery, pharmaceutical delivery mechanism, organic active ingredients, etc., can solve the problems of degradation of drugs, inability to provide information on the effect of autoclaving exposure on the particle size of methylprednisolone acetate, and the injectable suspension is one of the most difficult dosage forms to develop, etc., to achieve good stability, easy resus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
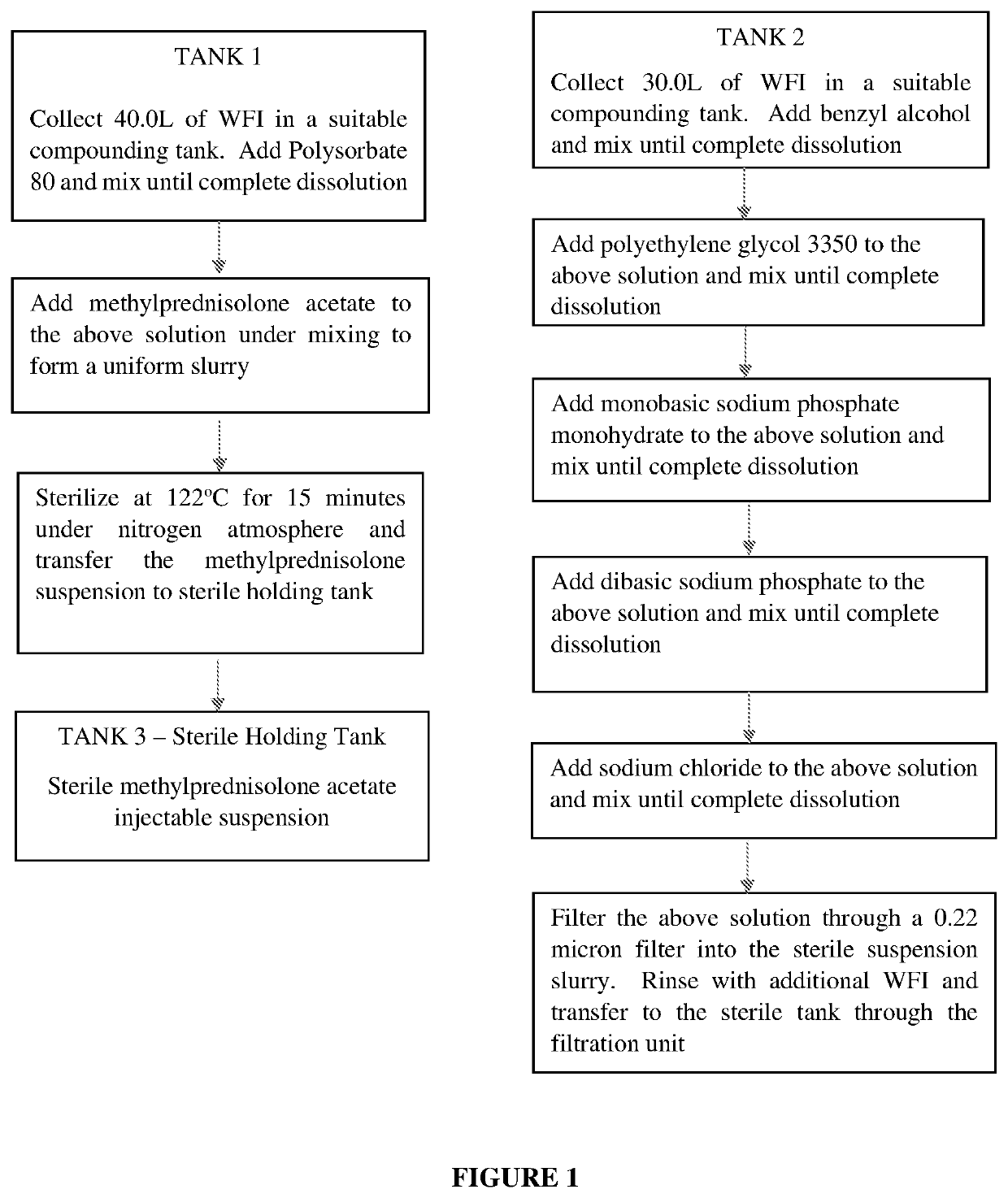
[0040]The present invention provides a process for preparation of a sterilized injectable suspension using moist heat sterilization. In particular, the invention relates to a process for sterilizing a water-insoluble steroid composition comprising moist heat sterilization of an aqueous slurry of a water-insoluble steroid in the presence of polysorbate.
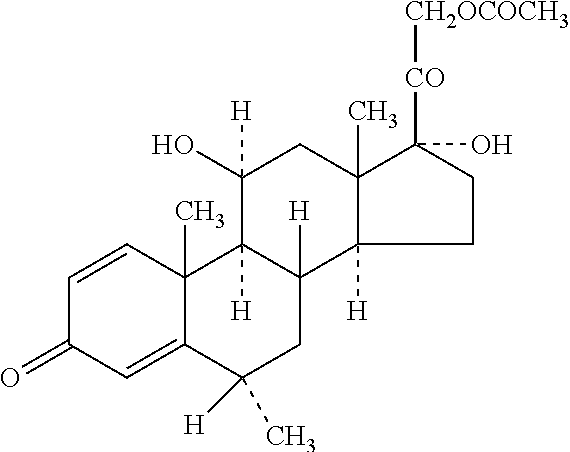
[0041]The term “water-insoluble steroid” is a steroid which is not completely dissolved at the concentration at which it is administered in an aqueous composition. Thus, depending upon the use and concentration, a steroid may be considered water-insoluble in one situation but not water-insoluble in another situation. While not intending to limit the scope of the invention in any way, the water-insoluble steroid referred to in the present invention relates to the pharmaceutically active agents or their pharmaceutically acceptable salts suitable for parenteral use and or normally supplied as suspension formulation. Such active agents inc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com