Nucleic acids for inhibiting expression of lpa in a cell
a technology of nucleic acids and lpa, which is applied in the field of nucleic acids for inhibiting the expression of lpa in a cell, can solve the problems of complex process, increased risk of suffering, and severe limitations of nucleic acid silencing trigger discovery, so as to prevent and reduce the risk of suffering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0437]A number of modified and conjugated siRNA molecules used for functional examples are shown here.
LPA-1038 Derivatives:
[0438]
GalNAc-LPA-1038-L1First strand(SEQ ID NO: 119, based on SEQ ID NO 5)OMeA-(ps)-FU-(ps)-OMeA-FA-OMeC-FU-OMeC-FU-OMeG-FU-OMeC-FC-OMeA-FU-OMeU-FA-OMeC-(ps)-FC-(ps)-OMeA 3′Second strand(SEQ ID NO: 120, based on SEQ ID NO SEQ ID NO 6)5′[ST23 (ps)]3 long trebler (ps)FU-OMeG-FG-OMeU-FA-OMeA-FU-OMeG-FG-OMeA-FC-OMeA-FG-OMeA-FG-OMeU-FU-(ps)-OMeA-(ps)-FU 3′GalNAc-LPA-1038-L6First strand(SEQ ID NO: 121, based on SEQ ID NO 5)OMeA-(ps)-FU-(ps)-OMeA-FA-OMeC-FU-OMeC-FU-OMeG-FU-OMeC-FC-OMeA-FU-OMeU-FA-OMeC-(ps)-FC-(ps)-OMeA 3′Second strand(SEQ ID NO: 122, based on SEQ ID NO 6)5′[ST23 (ps)]3 ST43 (ps)FU-OMeG-FG-OMeU-FA-OMeA-FU-OMeG-FG-OMeA-FC-OMeA-FG-OMeA-FG-OMeU-FU-(ps)-OMeA-(ps)-FU 3′
[0439]FN (N=A, C, G, U) denotes 2′Fluoro, 2′ DeoxyNucleosides
[0440]OMeN (N=A, C, G, U) denotes 2′O Methyl Nucleosides
[0441](ps) indicates a phosphorothioate linkage
[0442]ST23 and ST43 are as b...
example 2
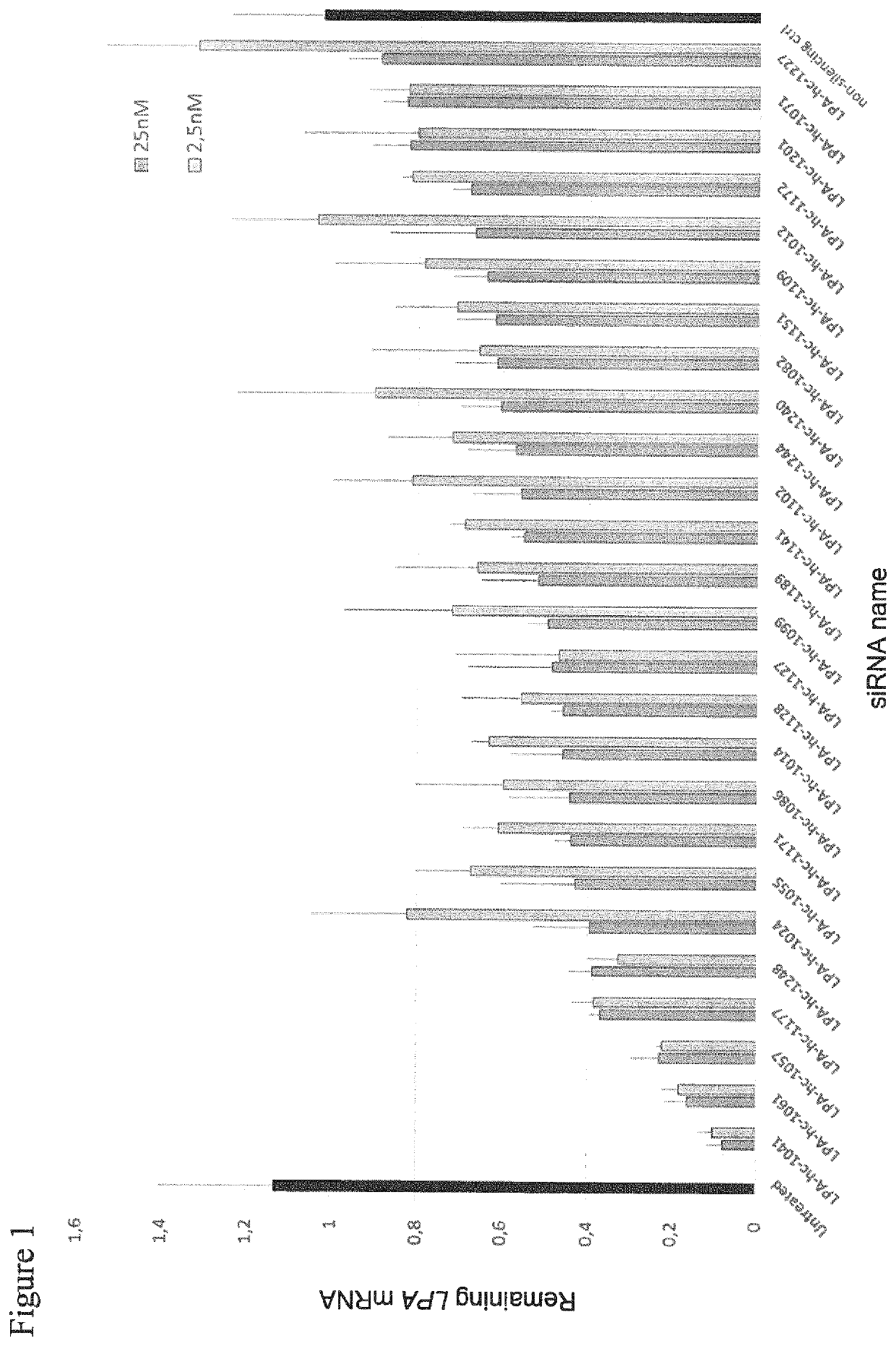
[0453]Screening of non-conjugated siRNA molecules (Table 1) for inhibition of LPA mRNA expression in human RT-4 cells.
[0454]Liposomal transfection complexes were prepared in triplicate at a ratio of 1.5 μl RNAiMax (ThermoFisher) / 80 pmol of the indicated siRNA molecules. The complex was diluted to the indicated concentrations of 2.5 nM and 25 nM, respectively (values represented pairwise as light and darker grey bars). RT4 human urinary bladder transitional cell papilloma cells expressing endogenously LPA were seeded at a density of 125.000 cells per well in 24-well format on top of previously plated transfection complexes (reverse transfection) at the indicated concentration. 24 hours after transfection total RNA was isolated using the Spin Cell Mini Kit 250 (Stratec). LPA mRNA levels were determined by qRT-PCR relative to PPIB mRNA expression in the respective samples as housekeeping transcript. Values were normalized to the amount of LPA mRNA detected in untreated cells (intraplat...
example 3
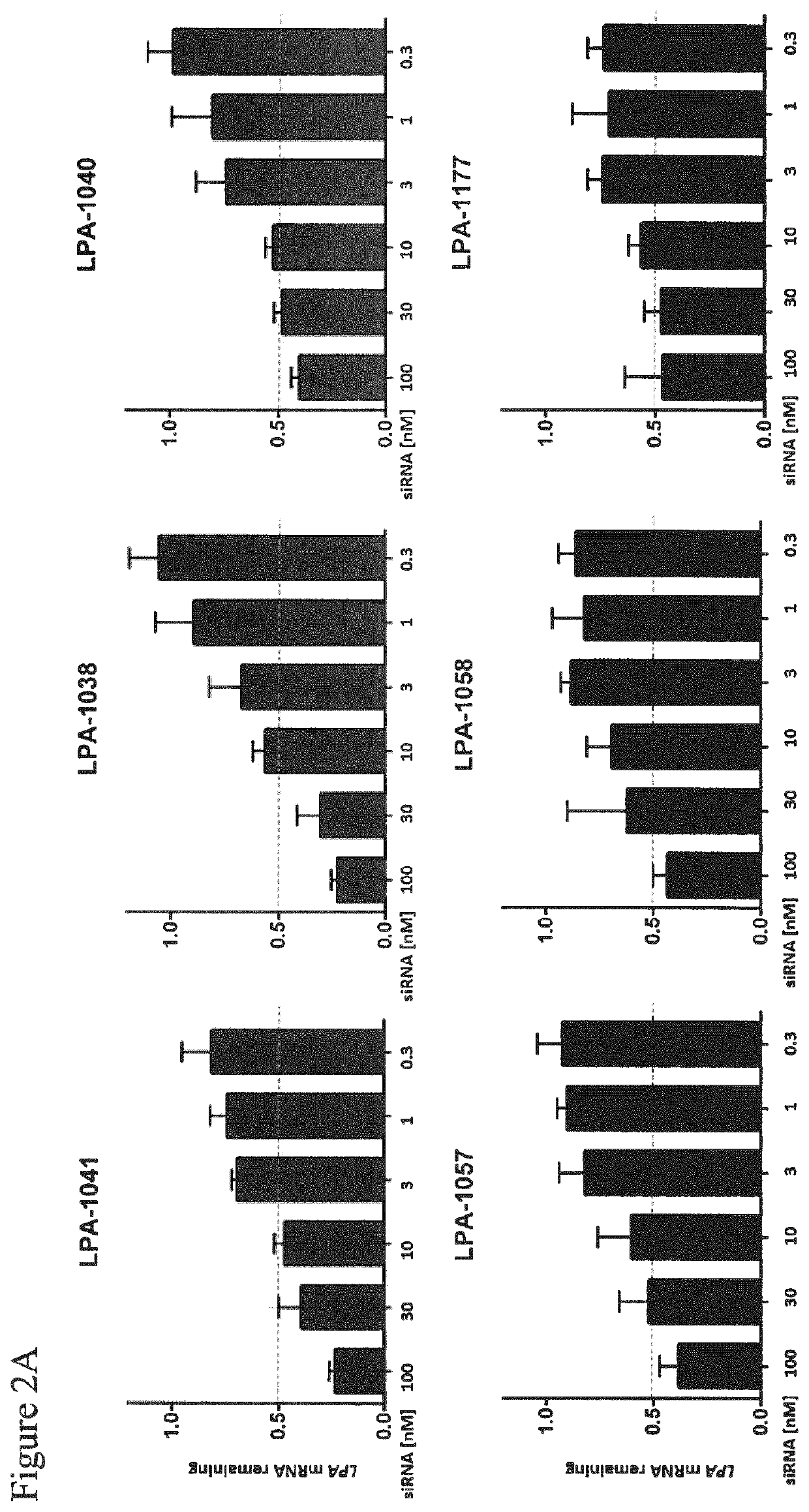
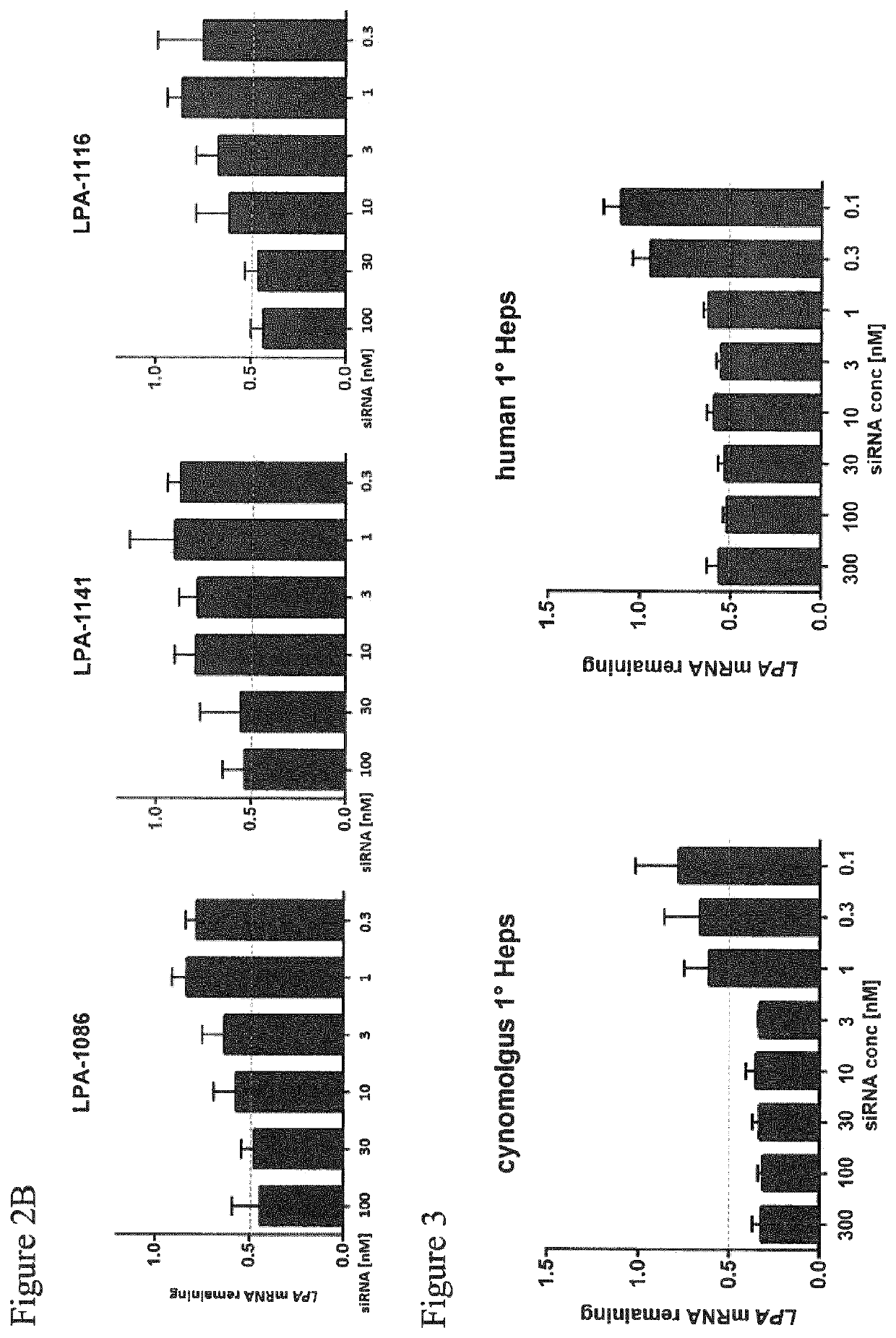
[0455]Dose response of non-conjugated LPA-targeting siRNA compounds on LPA mRNA expression in human RT-4 cells.
[0456]RT4 human urinary bladder transitional cell papilloma cells were reversely transfected as described above (Example 2) and treated at the indicated concentration (range 100 nM to 0.2 nM) with the different non-conjugated siRNA compounds (Table 1) as labeled. 24 h post transfection, total RNA was isolated using the Spin Cell Mini Kit 250 (Stratec). LPA mRNA levels were determined by qRT-PCR relative to PPIB mRNA expression in the respective samples as housekeeping transcript. Values were normalized to the amount of LPA mRNA detected in untreated cells. The bars represent the remaining LPA mRNA expression for each data point. Results are shown in FIG. 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com