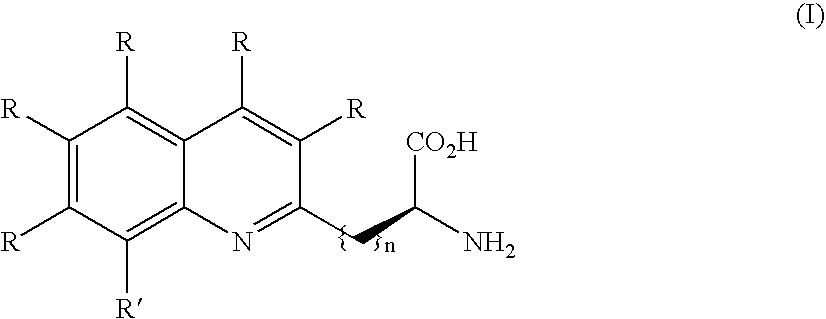
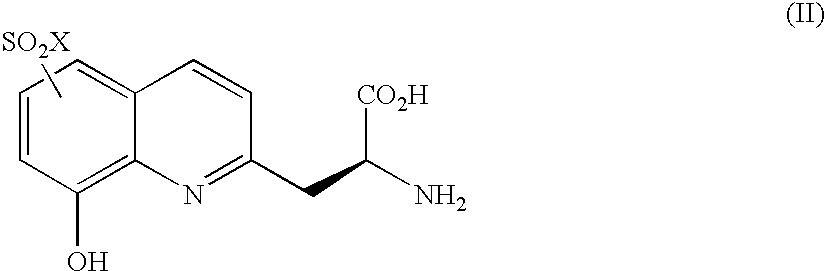
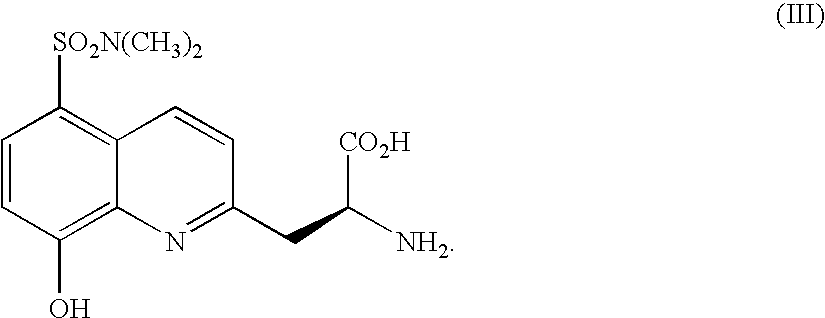
Fluorescence assay for kinase activity
a kinase activity and fluorescence assay technology, applied in the field of fluorescence assay for kinase activity, can solve the problems of affecting the recognition of kinases by certain kinases, affecting the reactivity of certain kinases, and few examples of sensors capable of such assays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Peptide Synthesis:
[0045]Peptides were synthesized using standard Fmoc amino acid protection chemistry on Fmoc-PAL-PEG-PS resin (0.22 mmol equiv.). Couplings of Fmoc-protected amino acids to the resin were carried out with 1-benzotriazolyoxytris (pyrrolidino) phophonium hexafluorophosphate (PyBOP), 1-hydroxybenzotriazole (H.OBt) and diisopropylethylamine (DIEA) or O-(7-azabenzotrazol-1-yl)-1,1,3,3-tetramethyl uranium hexafluorophosphate (HATU) and DIEA to generate the activated ester. The resin was swelled in dichloromethane (5 min.) then DMF (5 min.) prior to synthesis. All amino acids other than the Sox, phosphoserine, phosphothreonine and phosphotyrosine were added by the following representative procedure: removal of the Fmoc group (20% piperidine solution in DMF, 3×5 min.), wash (DMF, 5×1 min.), coupling (amino acid / PyBOP / DIEA, 6:6:6, 0.05 M in DMF, 45 min.), rinse (DMF, 2×1 min; DCM, 2×1 min.). To couple the Sox residue, double coupling with 2 equivalents each time was used (Fm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Emission wavelength | aaaaa | aaaaa |
| cExcitation wavelength | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com