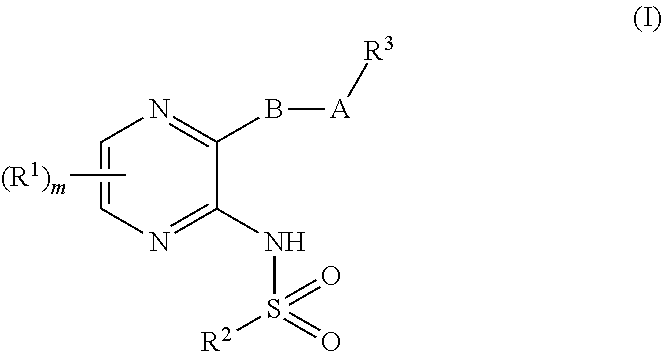
2,3-disubstituted pyrazinesulfonamides as CRTH2 inhibitors
a technology of pyrazine sulfonamide and pyrazine sulfonamide, which is applied in the direction of respiratory disorder, organic active ingredients, digestive system, etc., can solve the problems of low efficacy, indirect link between dp receptor activation and pgd2-stimulated eosinophil migration,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for the Synthesis of 2,3-Substituted Pyrazine Sulfonamide Derivatives of General Formula I, with A and Z as Above Defined (Schemes 1, 4, 5, 6, 7, 8, 9 and 10): N-{3-[4-(1H-indol-1-ylmethyl)phenyl]pyrazin-2-yl}-2-(trifluoromethyl)benzene-sulfonamide
[0239]
Method A:
[0240]To a solution of 1H-Indole (234 mg, 2.0 mmol, 1 eq) in dimethylformamide (10 mL) was added sodium hydride (80 mg, 2 mmol, 1 eq). After the evolution of hydrogen ceased, N-{3-[4-(chloromethyl)phenyl]pyrazin-2-yl}-2-(trifluoromethyl)benzene sulfonamide (intermediate 26) (854 mg, 2 mmol, 1 eq) in dimethylformamide (5 mL) was added and the reaction mixture heated to 80 degrees over 3 hours. The reaction was cooled, diluted with 30 mL of water and extracted with diethyl ether. The organic layer was dried over MgSO4, evaporated and purified by flash chromatography on silica gel eluting with AcOEt and cyclohexane to give pure N-{3-[4-(1H-indol-1-ylmethyl)phenyl]pyrazin-2-yl}-2-(trifluoromethyl)benzenesulfona...
example 2
2-chloro-N-{3-[4-({methyl[4-(trifluoromethoxy)phenyl]amino}-methyl)-phenyl]pyrazin-2-yl}benzene sulfonamide
[0243]
[0244]Following the general methods as outlined in Example 1 (Method B), starting from 2-chloro-N-{3-[4-(chloromethyl)phenyl]pyrazin-2-yl}benzenesulfonamide (Intermediate 8), and N-methyl-4-(trifluoromethoxy)aniline, the title compound was isolated as a yellow solid in 72% yield (99% purity by HPLC).
[0245]MS (ESI+): 550.1; MS (ESI−): 547.8.
example 3
N-(3-{4-[(2-ethyl-1H-benzimidazol-1-yl)methyl]phenyl}pyrazin-2-yl)-2-(trifluoromethyl)benzenesulfonamide
[0246]
[0247]Following the general method as outlined in Example 1 (Method B), starting from N-{3-[4-(chloromethyl)phenyl]pyrazin-2-yl}-2-(trifluoromethyl)benzenesulfonamide (Intermediate 9), and 2-ethylbenzimidazole, the title compound was isolated as a yellow solid in 63% yield (96% purity by HPLC).
[0248]MS (ESI+): 538.6; MS (ESI−): 536.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com