NOGO receptor-mediated blockade of axonal growth
A receptor and antibody technology, applied in the direction of receptors of neural mediators, growth factors/growth regulators, receptors/cell surface antigens/cell surface determinants, etc., can solve the mechanism of Nogo's inhibition of axonal growth has not yet been elucidated And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Example 1 - Identification of Nogo as a member of the Reticulon protein family
[0196] Axon regeneration in adult mammals is generally successful in the peripheral nervous system, but less so in the CNS. However, many classes of CNS axons extend longer distances in peripheral nerve grafts (Benfy and Aguayo (1982) Nature 296, 150-152). A comparison of CNS and peripheral nervous system (PNS) myelin revealed that CNS white matter selectively inhibits axonal outgrowth (Schwab and Thoenen (1985) J. Neurosci. 5, 2415-2423). Several components of CNS white matter - NI35, NI250 (Nogo) and MAG have been described to have inhibitory activity on axonal growth (Wang et al., (1999) Transduction of inhibitory signals by axonal growth cones. Neurobiology of Spinal Cord Injury Science", Kalb and Strittmatter (editors), Humanan Press; Caroni and Schwab, (1988) J. Cell Biol.106, 1281-1288; Spillmann et al., (1988) J. Biol. Chem.73, 19283-19293; McKerracher et al., (1994) Neuron 13, 80...
Embodiment 2
[0203] Example 2 - Anti-Nogo polyclonal antibody
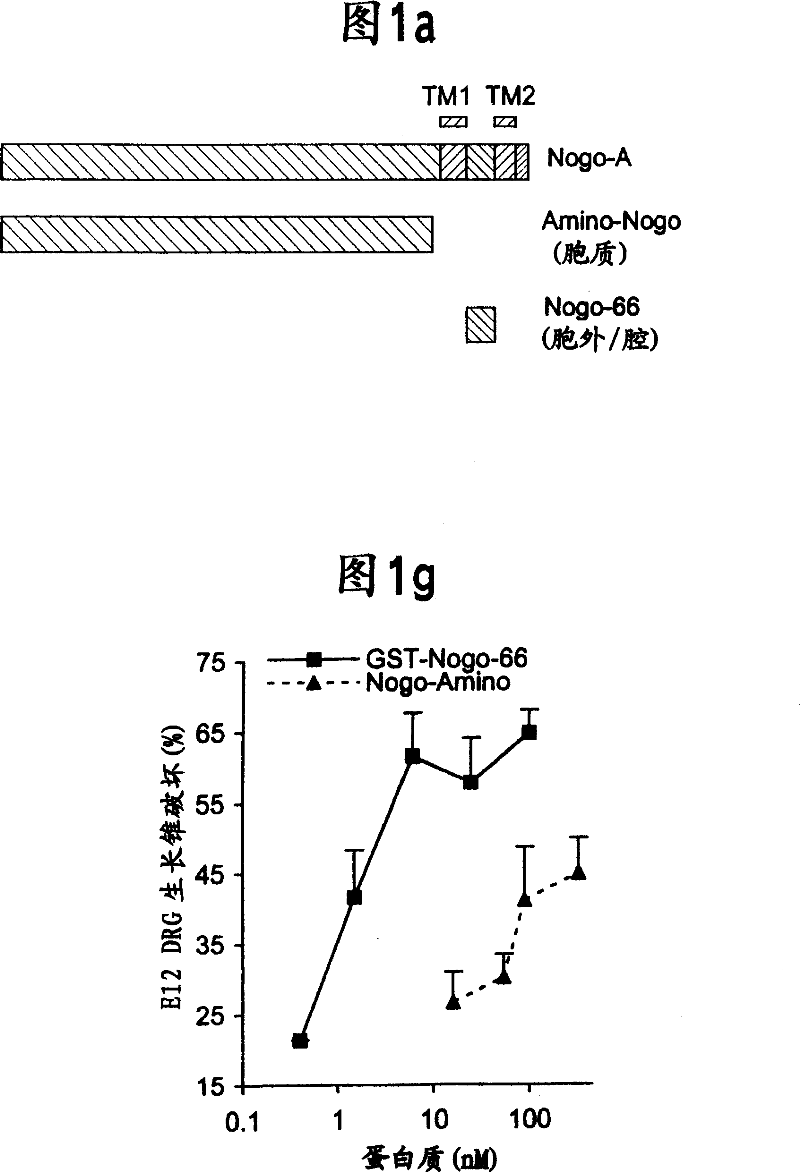
[0204] The predicted intramembrane topology of the two hydrophobic domains of Nogo suggests that the 66 amino acid residues between these segments are located in the luminal / extracellular membrane. For further studies, antisera were raised against this 66 amino acid domain.
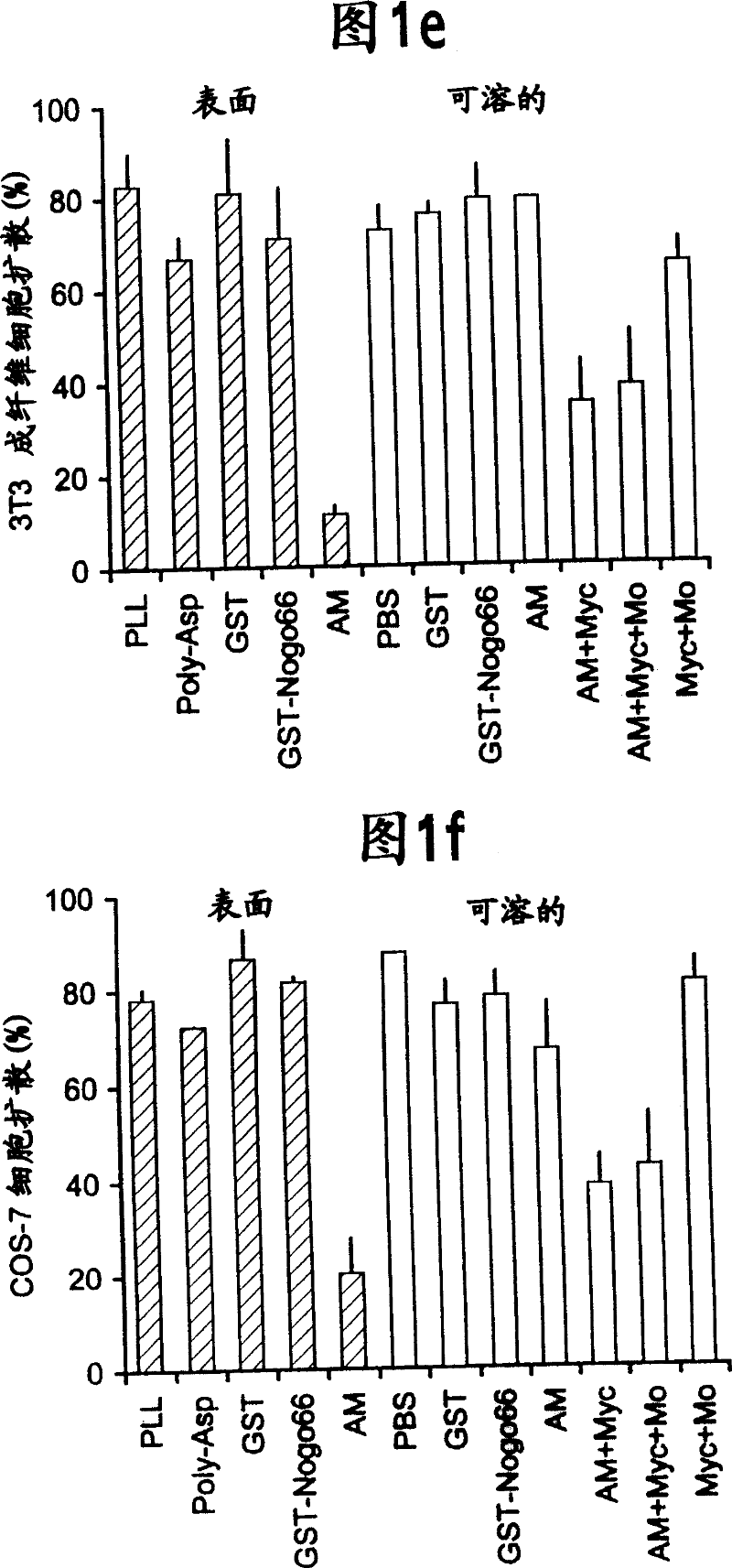
[0205] For antibody production and immunohistology, anti-Myc immunoblotting was performed using the 9E10 antibody as described by Takahashi et al., (1998) Nature Neurosci.1, 487-493 and Takahashi et al., (1999) Cell 99, 59-69 and immunohistology. GST-Nogo fusion protein was used as immunogen to generate anti-Nogo rabbit serum. Antibodies were affinity purified and used at 3 μg / ml for immunohistology and 1 μg / ml for immunoblotting. To estimate the specificity of the antisera, staining was performed in the presence of 0.1 mg / ml GST-Nogo protein. For live cell staining, cells were incubated with primary antibody dilutions in Hanks' balanced salt solution...
Embodiment 4
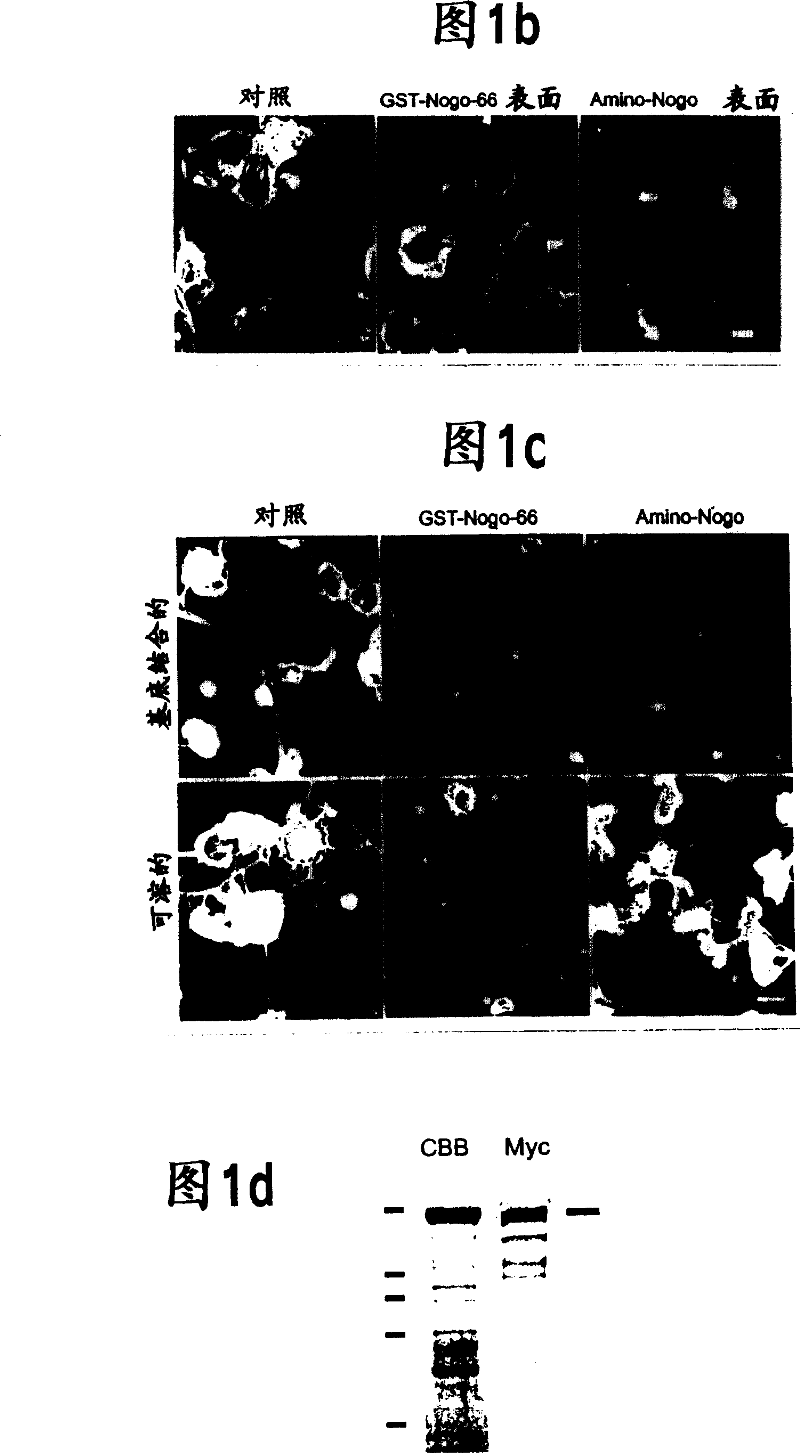
[0210] Example 4 - Nogo-mediated growth cone disruption
[0211] All experiments involving cell culture used the following methods. Chicken E10 and E12 DRG explants and dissociated neurons were cultured using the method described for E7 DRG culture (Takahashi et al., (1998) Nature Neurosci. 1, 487-493; Takahashi et al. (1999) Cell 99, 59-69; Goshima et al., (1995) Nature 376, 509-514; Jin and Strittmatter, (1997) J. Neurosci. 7, 6256-6263). NGF-differentiated PC12 cells were cultured as described (Strittmatter et al. (1994) J. Neurosci. 14, 2327-2338). Embryonic spinal cord explants (rat E10 or chicken E5) were cultured for 7-14 days in the presence of PDGF-AA to induce differentiation of some cells into mature oligodendrocytes (Vartanian et al., (1999) Proc. Natl. Acad. Sci. USA 96, 731-735). The procedure for the growth cone destruction assay was the same as that for the analysis of Sema3A-induced growth cone destruction (Takahashi et al., (1998) Nature Neurosci. 1, 487-4...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap