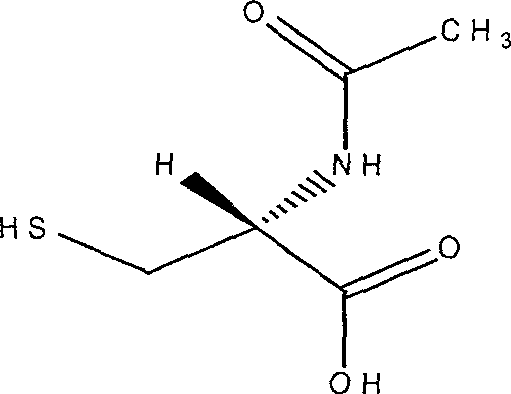
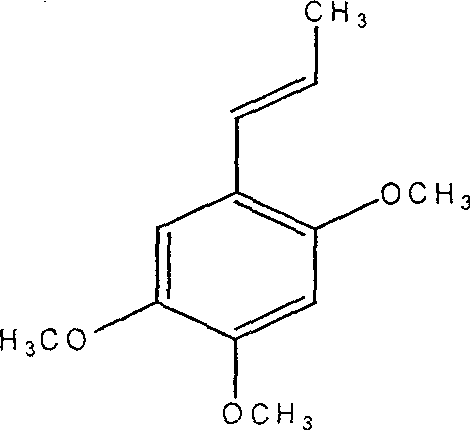
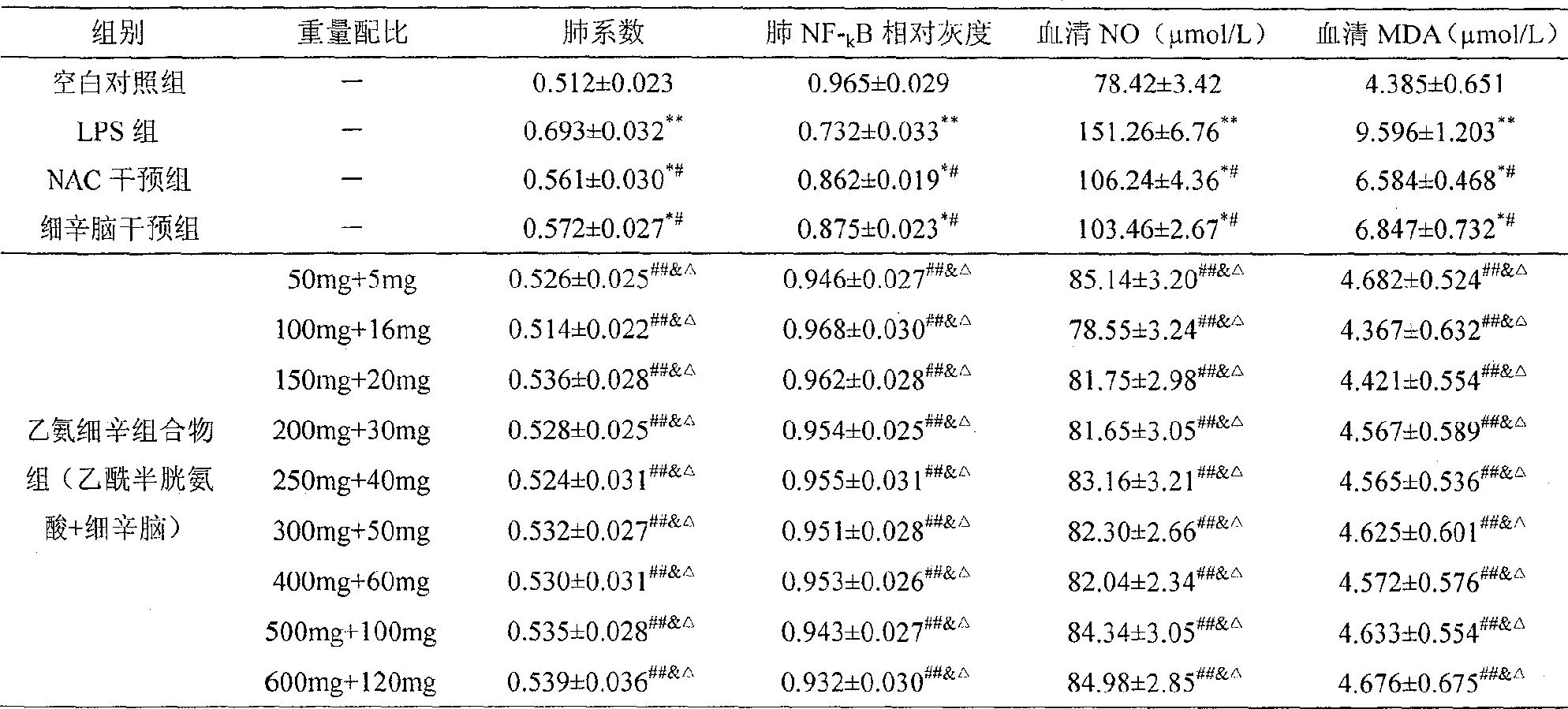
A medication composition of acetyl cysteine or a pharmaceutical salt thereof and asarin
A technology of acetylcysteine and its composition, applied in the field of medicine, to achieve wide application prospects, definite curative effect, and the effect of increasing sputum secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation of embodiment 1 etaminoxine composition powder injection
[0084] 1. Prescription:
[0085] Prescription of etaminoxine composition 1
[0086]
[0087] Prescription of etaminoxine composition 2
[0088]
[0089] 2. Specific steps:
[0090] 1) First, aseptically treat the containers used for liquid preparation, antibiotic glass bottles, rubber stoppers, etc.
[0091] 2) Weigh the raw materials and auxiliary materials according to the prescription quantity.
[0092] 3) Take sterile water for injection with a dosing volume of 80%, add mannitol and polysorbate 80, heat and stir to dissolve completely, add acetylcysteine and asarone, heat and stir to dissolve completely, add sterile water for injection to Full amount.
[0093] 4) Add activated carbon for needles with a dosing volume of 0.05%, heat and stir for 15 minutes.
[0094] 5) Filtration and decarburization. Measure and adjust the pH of the solution.
[0095] 6) Fine filtration throug...
Embodiment 2
[0100] Example 2 Preparation of Ethaminosarcoma Composition Aqueous Injection
[0101] 1. Prescription:
[0102] Prescription of etaminoxine composition 1
[0103]
[0104] Prescription of etaminoxine composition 2
[0105]
[0106] 2. Specific steps:
[0107] 1) Dispose of the pipes and containers used for liquid preparation one day in advance, and rinse them with fresh water for injection before use.
[0108] 2) Take the prescribed amount of ethanol and add an equal amount of water to mix, dissolve asarone, add 40% water for injection and polyethylene glycol 400 to dissolve acetylcysteine, mix the two, add water for injection to Full amount.
[0109] 3) Add activated carbon for needles with a dosing volume of 0.05%, and stir at 40°C for 15 minutes.
[0110] 4) Filtration and decarburization. Measure and adjust the pH of the solution.
[0111] 5) Fine filtration through a 0.45 μm microporous membrane.
[0112] 6) Check the clarity of the solution and test the ...
Embodiment 3
[0117] Example 3 Preparation of Ethaxarin Composition Sodium Chloride Infusion
[0118] 1. Prescription:
[0119] Prescription of etaminoxine composition 1
[0120]
[0121] Prescription of etaminoxine composition 2
[0122]
[0123] 2. Specific steps:
[0124] 1) Dispose of the pipes and containers used for liquid preparation the day before, and rinse them with fresh water for injection before use.
[0125] 2) Polysorbate 80 is made into a 20% solution with water for injection, acetylcysteine and asarone are added, heated and stirred to dissolve completely, and sodium chloride is completely dissolved with water for injection with a dosing volume of 40%. Combine the above solutions and add water for injection to the full amount.
[0126] 3) Add activated carbon for needles with a dosing volume of 0.05%, heat and stir for 15 minutes.
[0127] 4) Decarburization by sand filter rod filtration. Measure and adjust the pH of the solution.
[0128] 5) Fine filtration t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com