Technique for synthesizing air-dry type oxirene ester resin
A technology of epoxy vinyl ester and resin synthesis process, which is applied in the field of formulation and synthesis process of air-drying epoxy vinyl ester resin, which can solve the problem of reducing adhesion, clarity and gloss, unsuitable for industrialized large-scale production, resin Complicated production and other issues, to achieve the effect of good chemical resistance, high hardness, and high fullness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
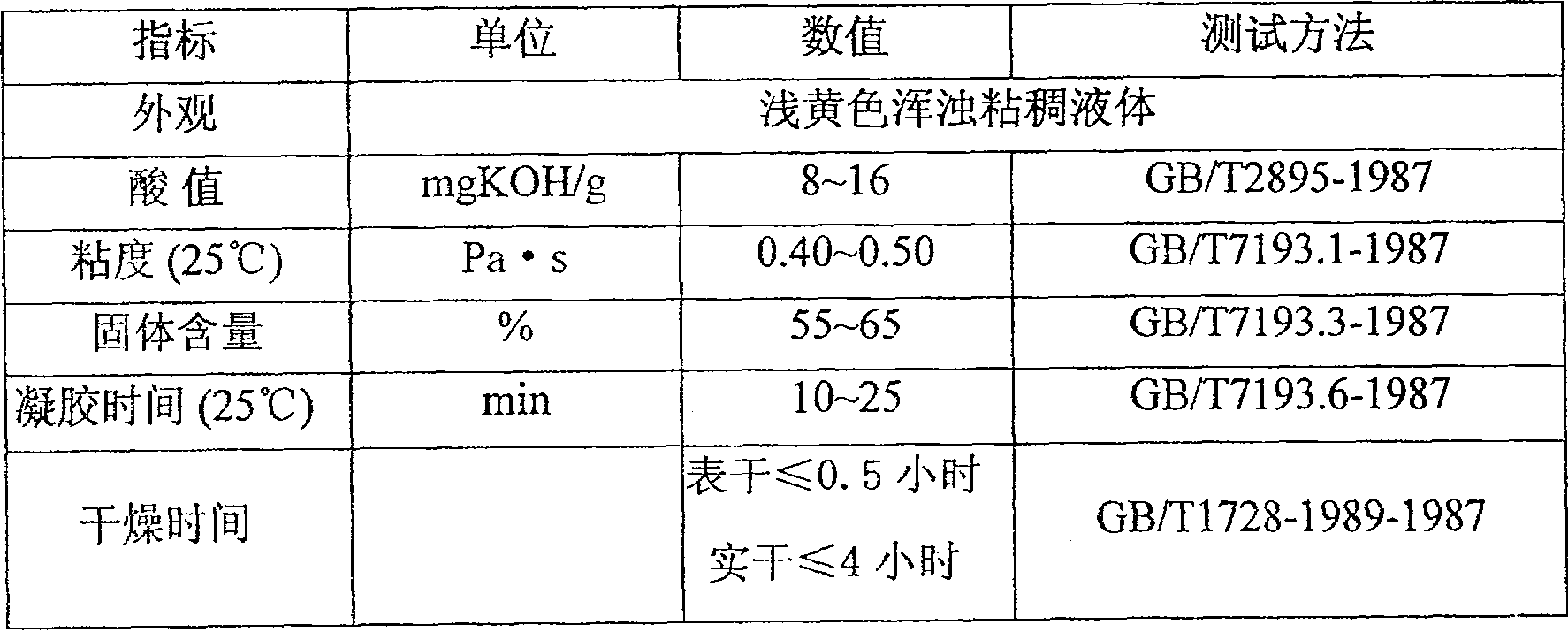
[0027] In the reactor, 170g of low epoxy equivalent bisphenol A epoxy resin (epoxy equivalent is 179~192g / mol) and high epoxy equivalent bisphenol A epoxy resin (epoxy equivalent is 455~500g / mol) mol) 170 g, 110 g of methacrylic acid, 0.8 g of benzyldimethylamine, and 0.15 g of tert-butylhydroquinone. In the case of nitrogen gas, gradually raise the temperature to 120°C, and keep the temperature until the measured acid value is less than 30mgKOH / g; cool down to below 110°C, add 260g of styrene, 20g of methyl methacrylate, stir well, when the temperature drops to 60°C Next, add 0.1 g of N-cyclohexyl-N'-phenyl-p-phenylenediamine, stir thoroughly, and finally cool to room temperature, and filter to obtain a light yellow viscous liquid.
Embodiment 2
[0029] 150g of bisphenol A type epoxy resin (epoxy equivalent is 185~196g / mol) of low epoxy equivalent, 200g of bisphenol A type epoxy resin of high epoxy equivalent (epoxy equivalent is 455~556g / mol) , 85g of acrylic acid, 0.38g of benzyltrimethylammonium chloride, and 0.08g of tert-butylcatechol are put into the device shown in Example 1, and the temperature is gradually raised to 130°C under the condition of feeding nitrogen gas, and the temperature is maintained until the acid is detected. The value is less than 30 mgKOH / g. Cool down to below 110°C, add 300g of styrene, stir well, when the temperature drops below 60°C, add 0.30g of N-isopropyl-N'-phenyl-p-phenylenediamine, stir well, and finally cool to room temperature, filter to obtain shallow Yellow viscous liquid.
Embodiment 3
[0031] 175g of bisphenol A type epoxy resin (epoxy equivalent is 185~208g / mol) of low epoxy equivalent, 230g of bisphenol A type epoxy resin of high epoxy equivalent (epoxy equivalent is 714~833g / mol) , 85g of acrylic acid, 1.6g of benzyltriethylammonium chloride, and 0.20g of p-benzoquinone are dropped into the device shown in Example 1, and the temperature is gradually raised to 140°C under nitrogen gas conditions, and the temperature is maintained until the acid value is less than 30mgKOH / g. Cool down to below 110°C, add 320g of styrene and 50g of divinylbenzene, stir well, when the temperature drops below 60°C, add 0.25g of N-(1,3-dimethyl)butyl-N'-phenyl-p-phenyl Diamine, fully stirred, finally cooled to room temperature, filtered to obtain light yellow turbid viscous liquid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| tack-free time | aaaaa | aaaaa |
| oxygen equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com