Compound containing cation radical of piperazidine, synthetic method, and application
A technology of cationic groups and compounds, applied in the field of compounds containing piperazine cationic groups and their synthesis and application, can solve problems such as simple synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
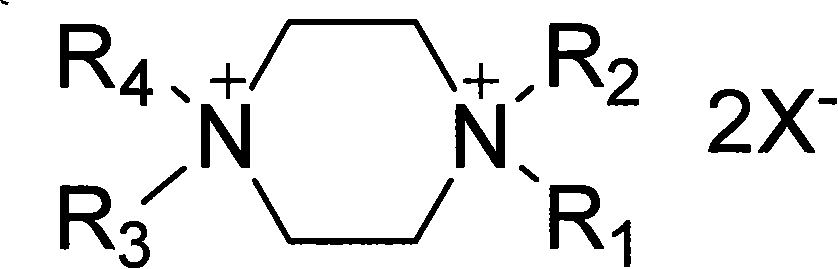
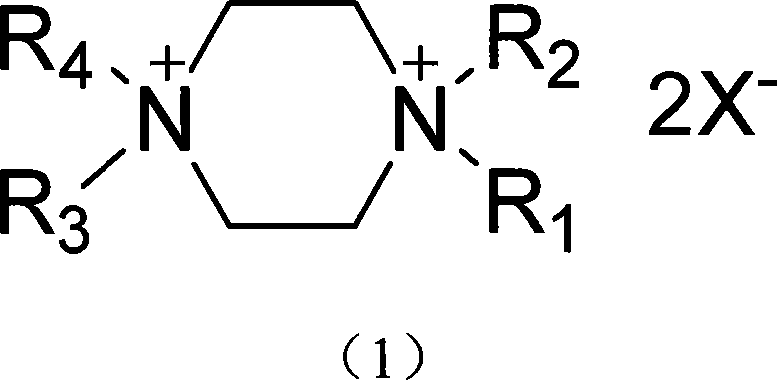
[0020] R in the above formula (1) 1, R 2 , R 3 and R 4 is butyl, X - The synthetic method that is the example compound of the present invention of fluoroborate anion comprises the following steps:
[0021] a. Add n-bromobutane to piperazine (the molar ratio of piperazine and n-bromobutane is 1:6), the solvent can be methanol, ethanol, propanol, isopropanol, N,N-dimethyl formamide, etc., heating reaction, the reaction temperature is 80°C, the pressure is 1-10 atmospheres, and the reaction time is 10-200 hours.
[0022] b, after the completion of the reaction (chromatographic detection), add ammonium fluoroborate (N, N, N', N'-tetrabutyl-piperazine ammonium bromide to ammonium fluoroborate molar ratio is 1: 2~ 6), the reaction temperature is 30°C, and the reaction time is 10-200 hours. The reaction product was purified to obtain N,N,N',N'-tetrabutylpiperazine ammonium fluoroborate.
[0023] same R 1 , R 2 , R 43 , R 4 for C 2 ~C 8 Alkyl, X is bromine or chloride ani...
Embodiment 1
[0026] N,N,N',N'-tetraethyl-piperazine ammonium bromide
[0027] Put 8.6 grams of piperazine, 100 grams of bromoethane and 150 grams of solvent methanol in the reaction device, the reaction temperature is 50 ° C, the pressure is 4 atmospheres, and the reaction time is 200 hours. After the reaction is completed (chromatographic detection), the solvent is evaporated . After the reaction product is purified, N,N,N',N'-tetrabutyl-piperazine ammonium bromide can be obtained, which is stable to air, and the yield is 93%.
[0028] m.p.: 50°C
[0029] High resolution mass spectrometry: [M-Br] + =200
[0030] 1 HNMR (D 2 O, δ / ppm relative to TMS): 3.625 (t, 8H), 3.281 (t, 8H), 1.251 (t, 12H).
Embodiment 2
[0032] N,N,N',N'-tetraisobutyl-piperazine ammonium chloride
[0033] Put 8.6 grams of piperazine, 180 grams of 2-chlorobutane and 150 grams of solvent ethanol in the reaction device, the reaction temperature is 80 ° C, the pressure is 1 atmosphere, and the reaction time is 100 hours. After the reaction (chromatographic detection), steam To solvent. The reaction product can be purified to obtain N,N,N',N'-tetrabutyl-piperazine ammonium chloride, which is air-stable, and the yield is 94%.
[0034] m.p.: 56°C
[0035] High resolution mass spectrometry: [M-Cl] + =312
[0036] 1 HNMR (D 2 O, δ / ppm relative to TMS): 3.332 (m, 4H), 3.615-3.618 (s, 8H), 1.714 (m, 8H), 1.332 (t, 12H), 0.964 (t, 12H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com