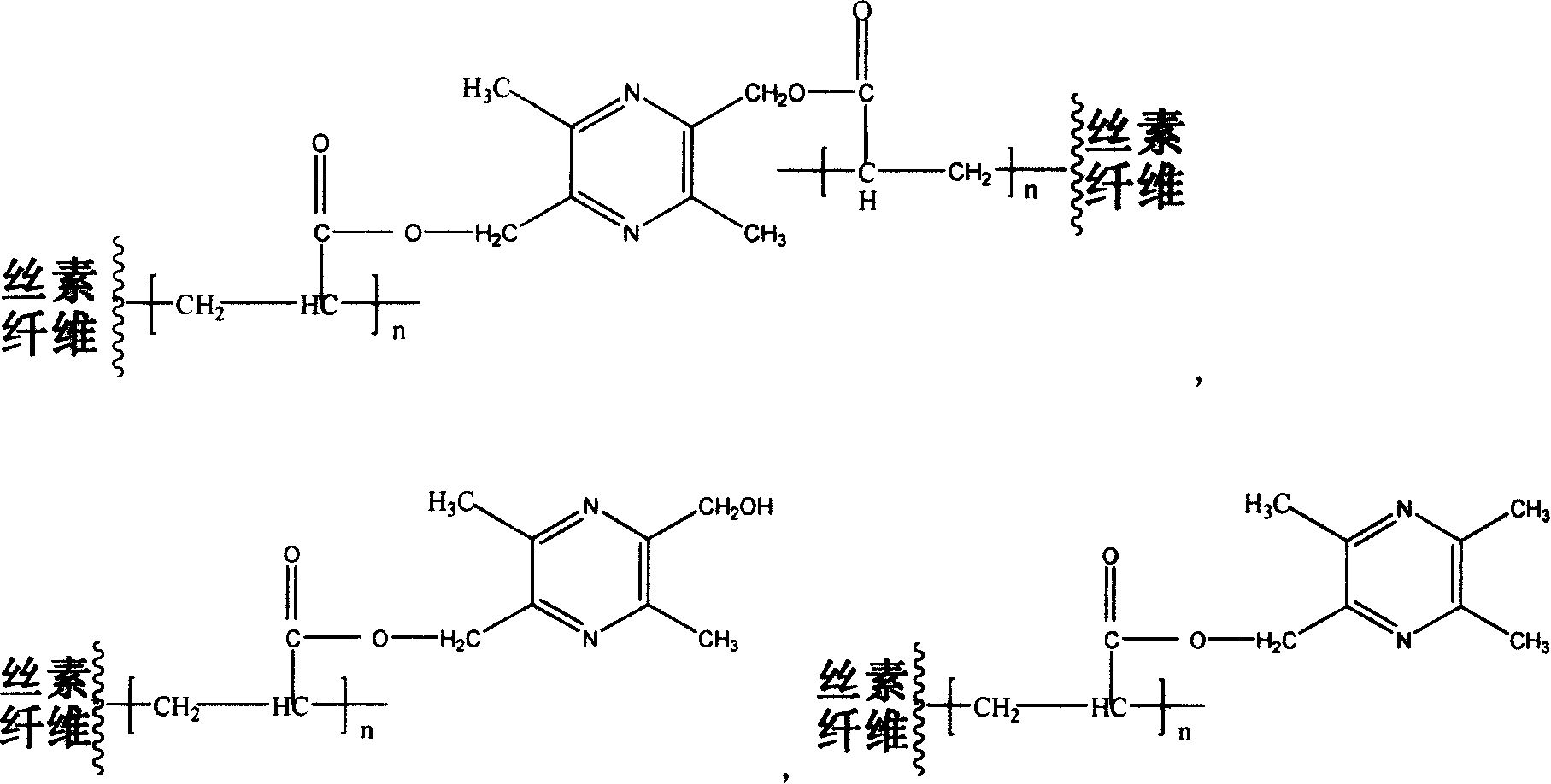
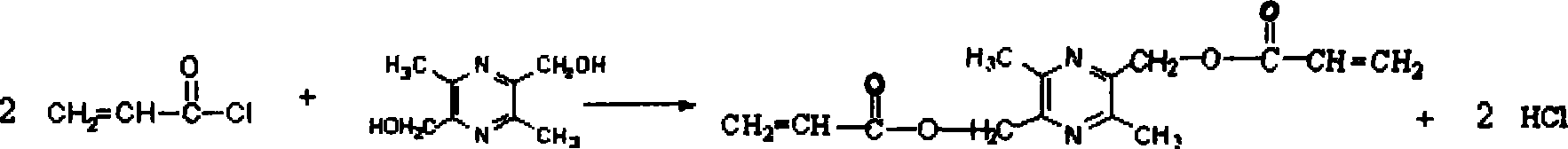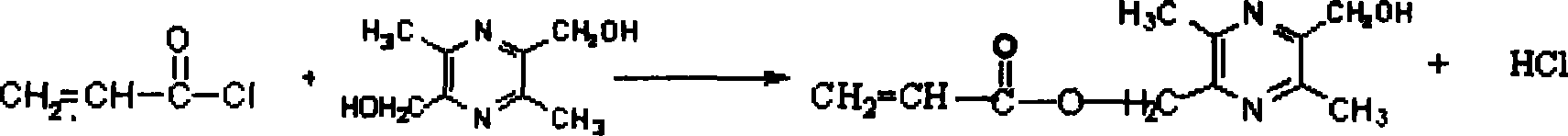Tetramethylpyrazine derivatives modified silk fibroin protein fiber anti-coagulant material and its preparation method
A technology of silk fibroin and ligustrazine, applied in the field of biomedical materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0021] (1) Preparation of 2,5-diacryloyloxymethylene-3,6-dimethylpyrazine (PZOE2)
[0022] Synthesize 2,5-dimethylol-3,6-dimethylpyrazine (TMPD2) according to literature (see Chinese patent, application number 200410042876.9[2]), with a melting point of 76-83°C. Then in a 100ml round bottom flask, 1.23g TMPD2 was dissolved in a mixed solution of 15ml DMF and 1.36ml pyridine, 1.2ml acryloyl chloride was dissolved in 15ml DMF, and was added dropwise into the flask from a constant pressure funnel, and the dropwise addition was completed in one hour. The reaction was continued for 24 hours and left to stand for 48 hours. After filtration, the reaction solution was poured into a large amount of glacial ether, and a pale yellow precipitate appeared, which was collected after centrifugation to obtain an orange-red substance. The remaining ether was evaporated at room temperature. Obtain 2,5-diacryloyloxymethylene-3,6-dimethylpyrazine, FTIR (KBr): 1731cm -1 (C=O, ester carbonyl), 1...
preparation example 2
[0031] (1) Preparation of 2-acryloyloxymethylene-5-hydroxymethyl-3,6-dimethylpyrazine (PZOHOE)
[0032] In a 100ml round bottom flask, dissolve 1.23g TMPD2 in a mixed solution of 15ml DMF and 0.7ml pyridine, and dissolve 0.7ml acryloyl chloride in 15ml DMF, and drop them into the flask from a constant pressure funnel. After one hour, continue the reaction 24 hours, let stand for 48 hours. After filtration, the reaction solution was poured into a large amount of glacial ether, and a pale yellow precipitate appeared, which was collected after centrifugation to obtain an orange-red substance. The remaining ether was evaporated at room temperature. Obtain 2-acryloyloxymethylene-5-hydroxymethyl-3,6-dimethylpyrazine, FTIR (KBr): 1735cm -1 (C=O, ester carbonyl), 1489cm -1 (-N=C-, pyrazine ring), at 1172cm -1 (C-O-C).
[0033] (2) Preparation of silk fibroin fibers.
[0034] Repeat the steps of Preparation Example 1
[0035] (3) Chemical modification of silk fibroin fibers
[...
preparation example 3
[0040] (1) Preparation of 2-acryloyloxymethylene-3,5,6-trimethylpyrazine (PZOE)
[0041] Synthesize 2-hydroxymethyl-3,5,6-trimethyl according to the literature (see "Research on the Structure Modification of Ligustrazine and Its Anticoagulant Property" Beijing Institute of Technology Master's Degree Thesis (2004): Author: Xia Chengjian [3]) Pyrazine (TMPD1), melting point 70~72℃. Dissolve 0.91g TMPD1 in a mixed solution of 11.5ml DMF and 0.485ml pyridine, dissolve 0.487ml acryloyl chloride in 11.5ml DMF, and drop them into the flask from a constant pressure funnel; after one hour, continue to react for 24 hours, Set 48h. After filtration, the reaction solution was poured into a large amount of glacial ether, and a pale yellow precipitate appeared, which was collected after centrifugation to obtain an orange-red substance. The remaining ether was evaporated at room temperature. Obtained 2-methacrylate-5-hydroxymethyl-3,6-dimethylpyrazine, FTIR (KBr): 1731cm -1 (C=O, ester c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap