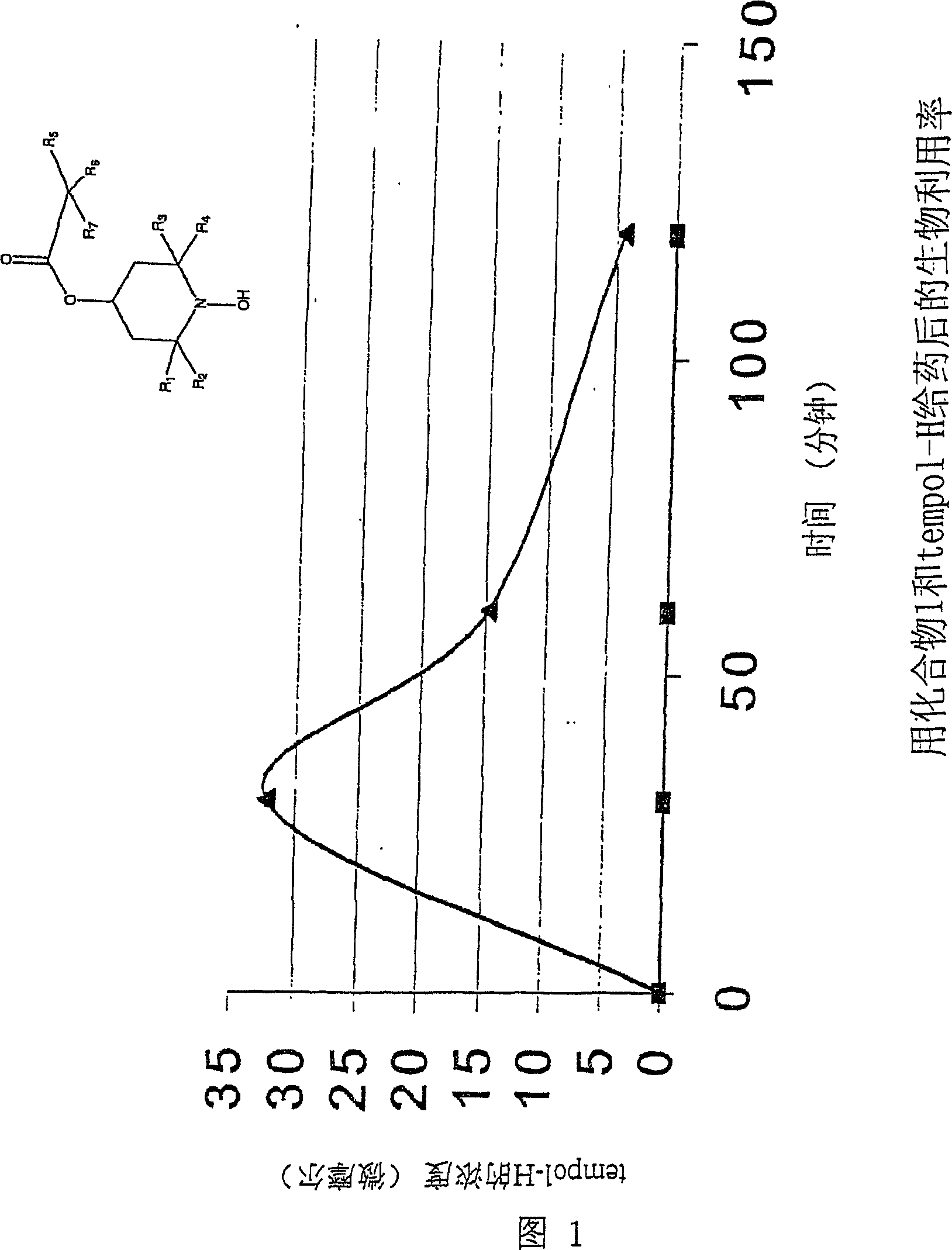
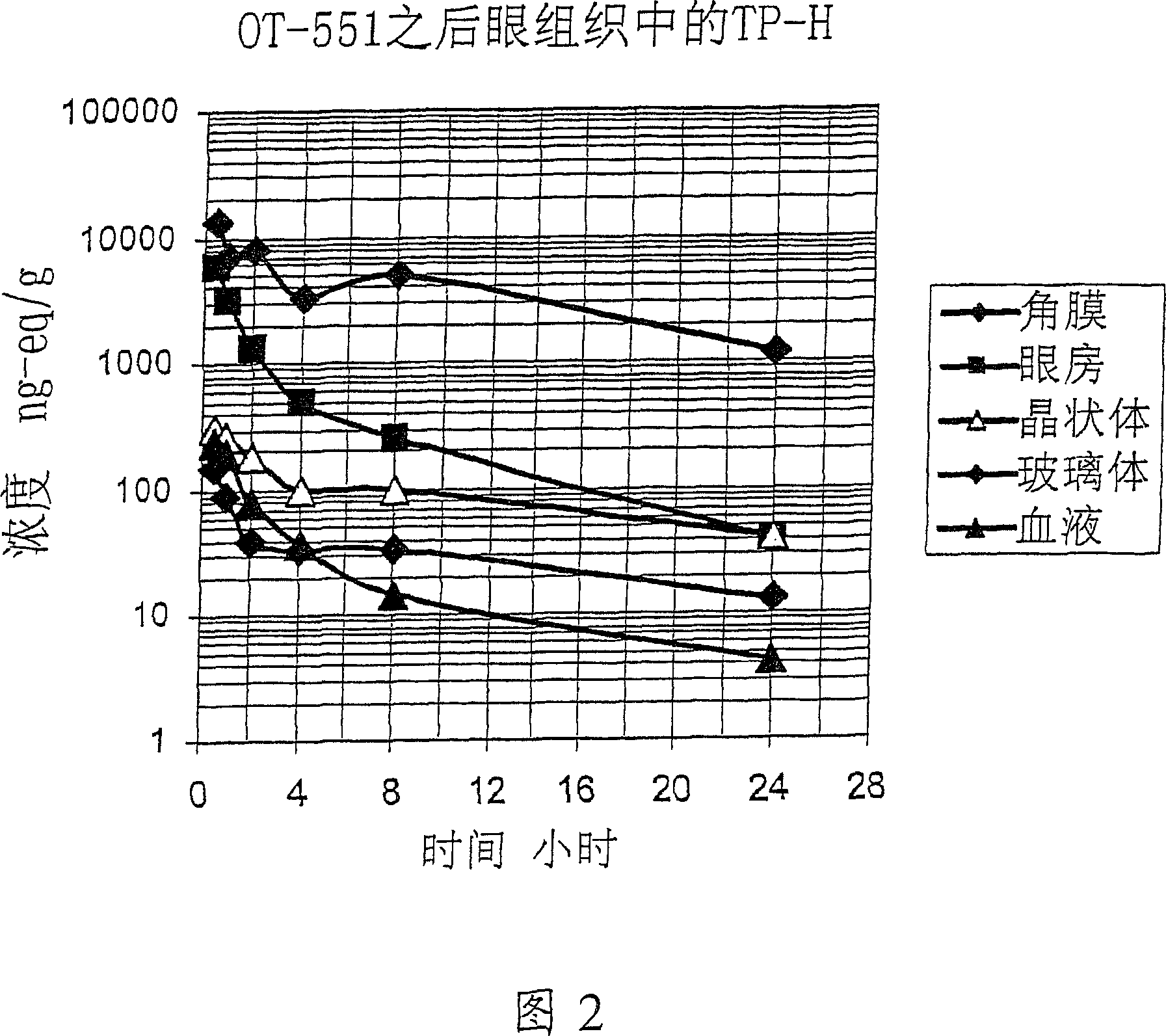
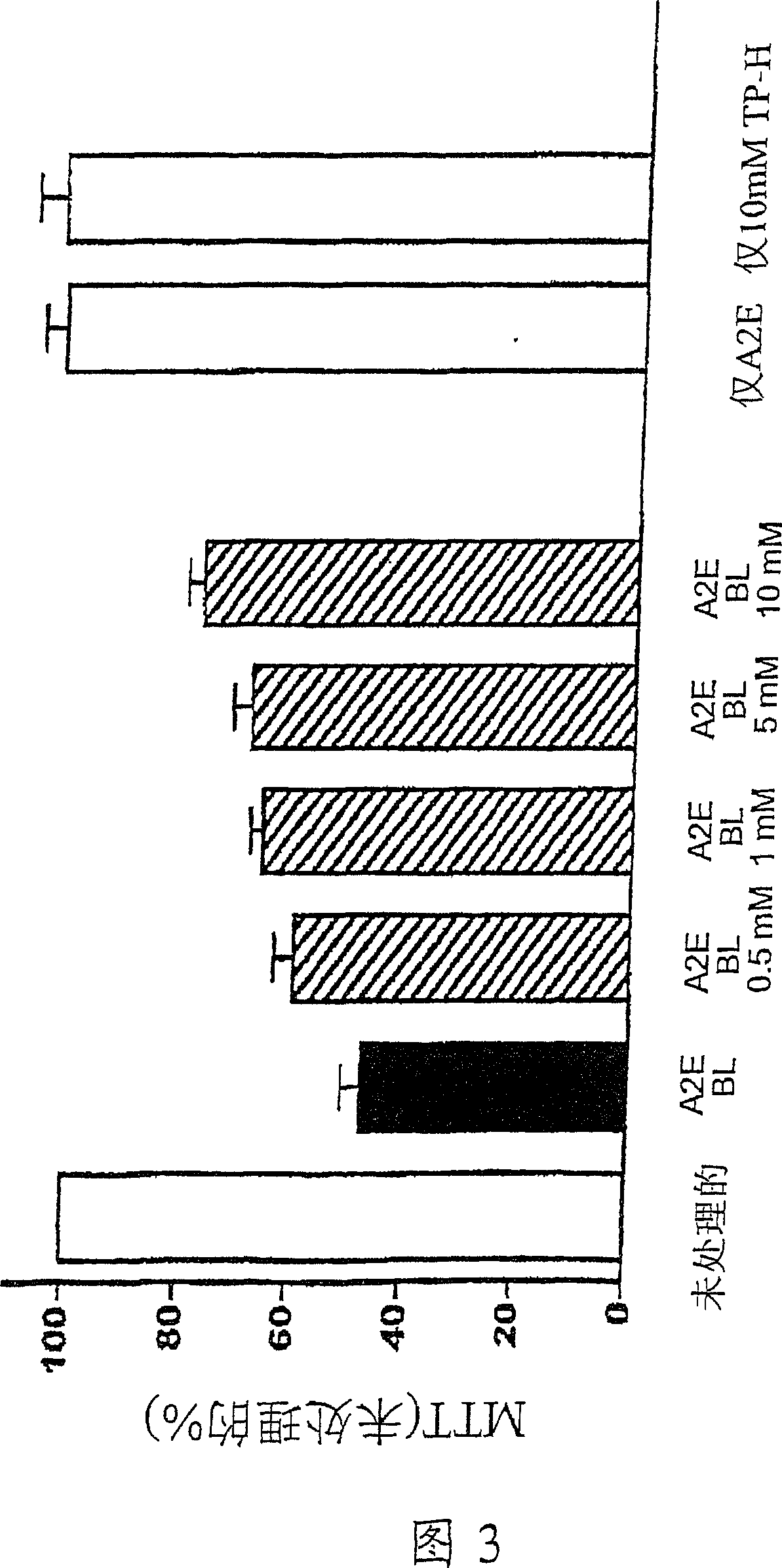
Amelioration of cataracts, macular degeneration and other ophthalmic diseases
A technology for macular degeneration, diseases, applied in the direction of skin diseases, sensory diseases, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1: Determination of the stability of ester compounds in aqueous humor
[0114] Methods: A 0.1-0.5% solution of the ester compound was prepared in a buffer (pH 4.5-5.0) containing DPTA or EDTA. The solution was filled into brown glass vials, which were sealed and placed in a temperature-controlled container maintained at 40°C. Sample vials were removed periodically and stored at 0-5°C until analyzed by HPLC, GC or GC / MS and found to be stable after 3 months under these conditions.
[0115] In order to be effective as an anti-cataract drug, the agent must be able to penetrate the lens. This can be included in methods of selecting anti-cataract compounds. The method for tempol-H is described as follows:
Embodiment 2
[0116] Example 2: Drug Permeation of Organ Cultured Rat Lenses
[0117] In contrast to drugs previously tested as anti-cataract agents, tempol-H and tempol have a remarkable ability to penetrate the lens tissue from the surrounding fluid. The assay described in this section measures the time period, active compound concentration and compound distribution in the lens following incubation with the rat lens under the organ culture conditions.
[0118] METHODS: Rat lenses were cultured as follows: Rat lenses were obtained from Sprague-Dawley rats. The lens was incubated in a 24-well culture plate filled with modified TC-199 medium, and placed in an atmosphere containing 95% air / 5% CO 2 atmosphere in a 37 °C incubator. The lenses were incubated in 2 ml of medium adjusted to 300 milliosmosm (mOsm). The lenses were incubated for 1-24 hours in medium containing 4.0 mM tempol-H or 4.0 mM oxidized form of tempol. At appropriate times, the lenses were removed from the medium, blotted...
Embodiment 3
[0122] Example 3: 1-oxyl-4-(3'-ethoxy-2',2'dimethyl)propanecarbonyloxy-2,2,6,6-tetramethylpiperidine
[0123]
[0124] In dry DMF (10 mL), 3-ethoxy-2,2-dimethylpropionic acid (750 mg, 7.13 mmol; prepared according to the method described in J.Org.Chem., 38, 2349, 1975, To the stirred solution, the contents of which are incorporated herein by reference), was added 1,1'-carbonyldiimidazole (1.27 g, 7.84 mmol) in small proportions. Vigorous gas evolution was observed. The solution was heated at 100°C for 1 hour. To this mixture was added tempol (900 mg, 5.23 mmol) and 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU) (800 mg, 5.26 mmol) and heating was continued for 12 hours. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in ethyl acetate (100 mL) and washed with 1N HCl, saturated NaHCO 3 Washed successively with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to give a red solid (1.48 g). It was subjected to silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com