Diastereoisomers of 4-hydroxyisoleucine and uses thereof
A technology of uses, compounds, applied in the field of isomers and their lactones, pharmaceutically acceptable salts and prodrugs, capable of solving problems such as no one has proven
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Example 1 : General procedure for the preparation of 4-hydroxyisoleucine isomers
[0119] A) General Experimental Procedures
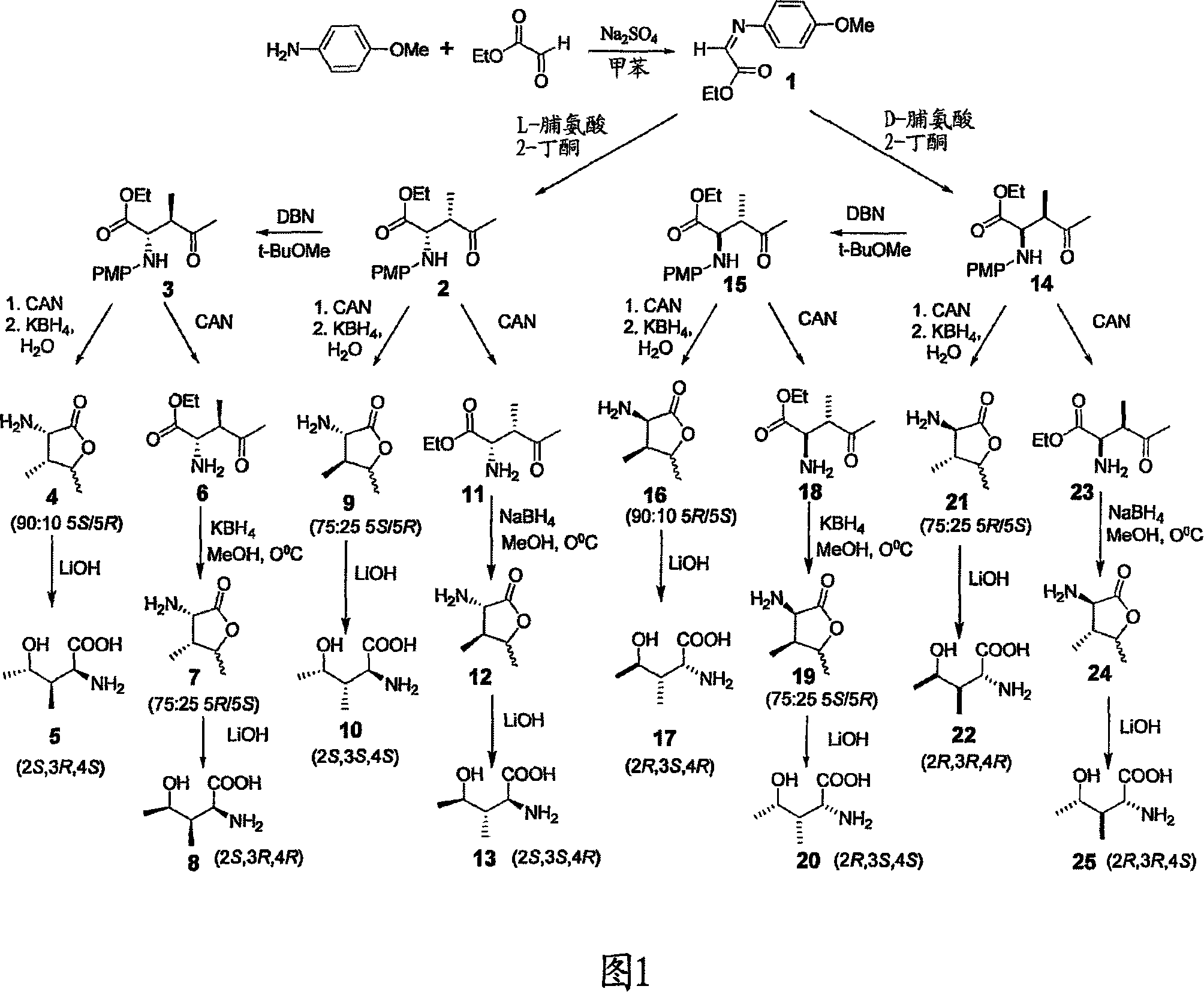
[0120] Figure 1 shows the synthetic schemes of 8 different configurational isomers (SRS, SRR, SSS, SSR, RSR, RSS, RRR and RRS) of 4-hydroxyisoleucine. The imine intermediate 1 was prepared from p-anisidine and ethyl glyoxylate (Cordova et al., A highly enantioselective amino acid-catalyzed route to functionalized alpha-amino acids ), J. Am. Chem. Soc. 124:1842-43, 2002). Reaction of imine 1 with 2-butanone in the presence of L-proline as catalyst followed by silica gel chromatography gave 2S,3S isomer 2. Epimerization at C-3 was achieved using 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) to give 2S,3R isomer 3. The (2S, 3R, 4S), (2S, 3R, 4R), (2S, 3S, 4S) and (2S, 3S, 4R) isomers of 4-hydroxyisoleucine are obtained from 2 or 3 as follows:
[0121] Deprotection of the amine moiety of 3 (removal of p-methoxyphenyl) using ceric ammonium nitrate (...
Embodiment 2
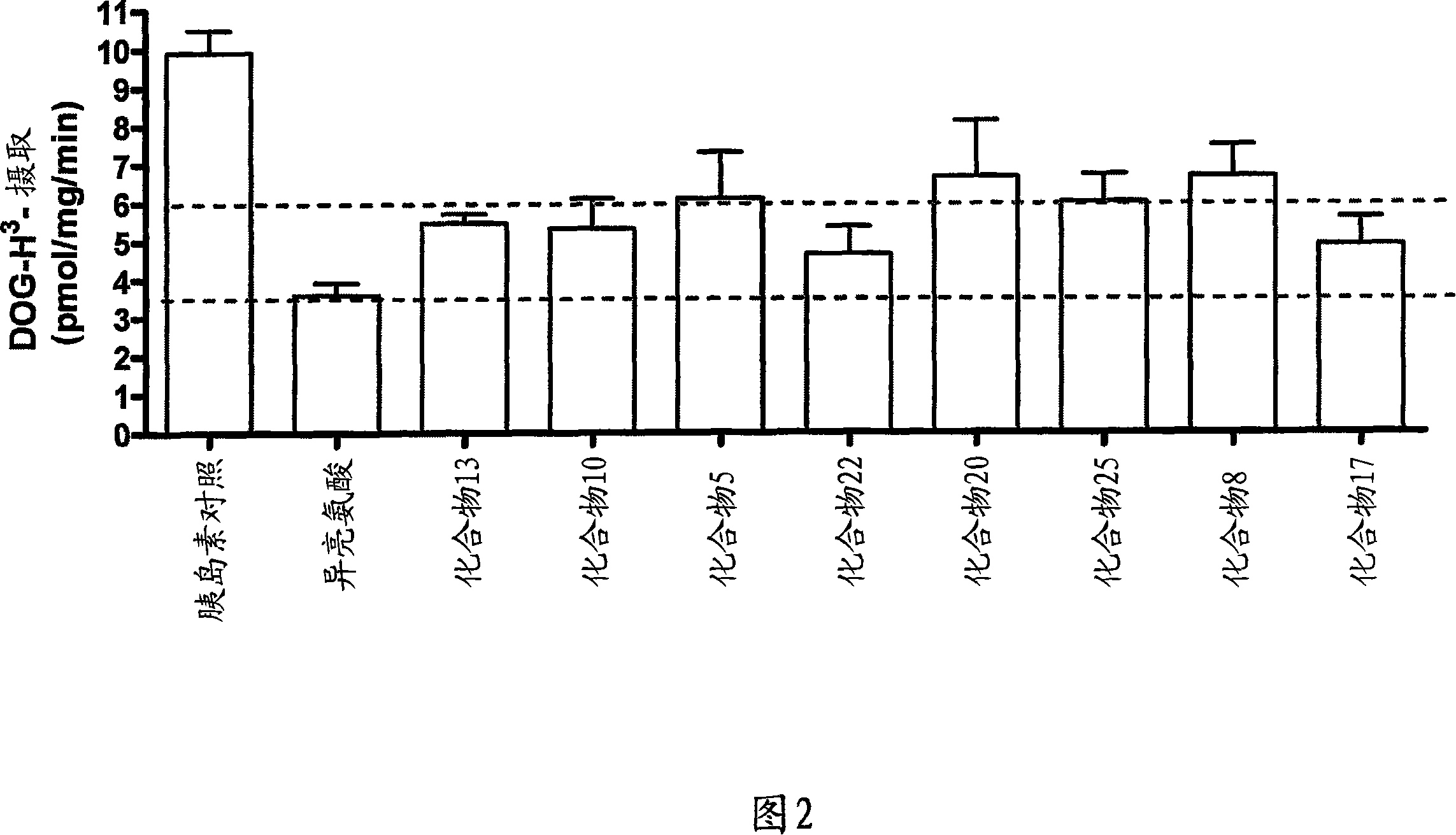
[0154] Example 2: Effect of Conformational Isomers of (2S,3R,4S)-4-Hydroxyisoleucine on Differentiation of 3T3-L1 Fat Stimulation of glucose uptake by cells
[0155] 3T3-L1 adipocytes (ATCC; CI-173) were cultured in 12-well tissue culture plates for 3 days to reach confluency (Lakshmanan et al., Analysis of insulin-stimulated glucose uptake in differentiated 3T3-L1 adipocytes. glucose uptake in differentiated3T3-L1 adipocytes). Diabetes Mellitus: Methods and Protocols, Saire Ozena, ed., Humana Press Inc., Tonowa, New Jersey 97-103, 2003). The medium was removed and replaced with differentiation medium (Green and Meuth, Cell 3: 127-133, 1974; Madsen et al., Biochem. J. 375: 539-549, 2003), and the cells were cultured for an additional 9 days . The differentiation status was confirmed by visual inspection. Cell starvation was performed for 5 hours by replacing the differentiation medium with medium lacking fetal bovine serum. During the last 30 minutes of the starvation p...
Embodiment 3
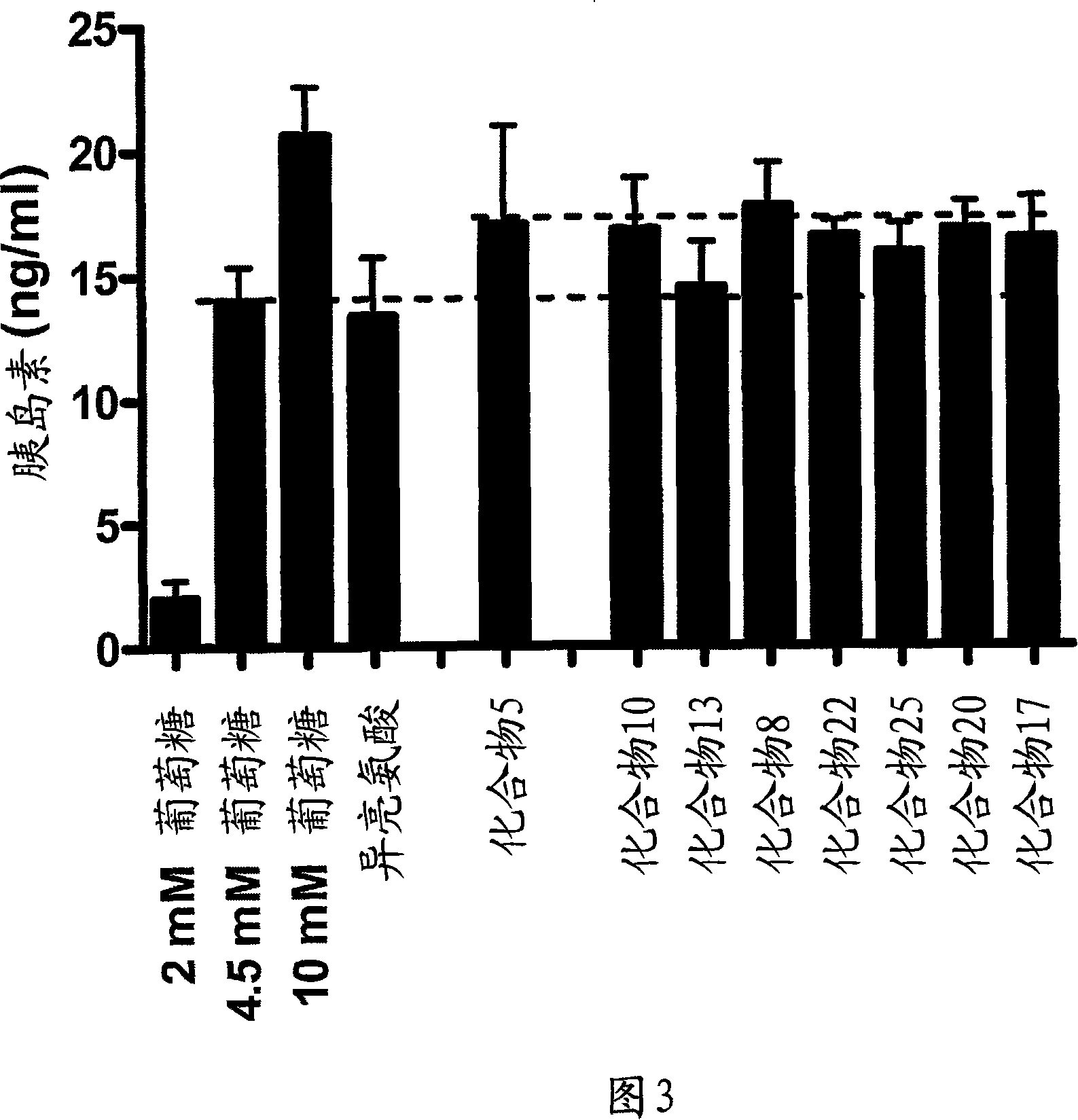
[0157] Example 3: Conformational isomers of (2S, 3R, 4S)-4-hydroxyisoleucine on islets of INS-1 cells glucose-dependent stimulation of hormone secretion
[0158] The insulinotropic effect of 4-hydroxyisoleucine configurational isomers on INS-1 cells was tested in a blind manner. Briefly, cells were 2 × 10 5 were plated in 12-well plates and cultured for 2 days in RPMI containing 10% fetal bovine serum and 11 mM glucose. The medium was removed 3 days after plating and replaced with RPMI containing 3 mM glucose and 10% fetal bovine serum. Cells were cultured for an additional 24 hours. On day 4 after plating, the medium was removed and replaced with Krebs-Ringer bicarbonate buffer containing 2 mM glucose. Cells were incubated for 30 minutes and the buffer was removed and replaced with Krebs-Ringer bicarbonate buffer containing 4.5 mM glucose and the optical isomer at a concentration of 0.5 mM. Cells were incubated for 1 hour. Basal insulin secretion was determined by cu...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap