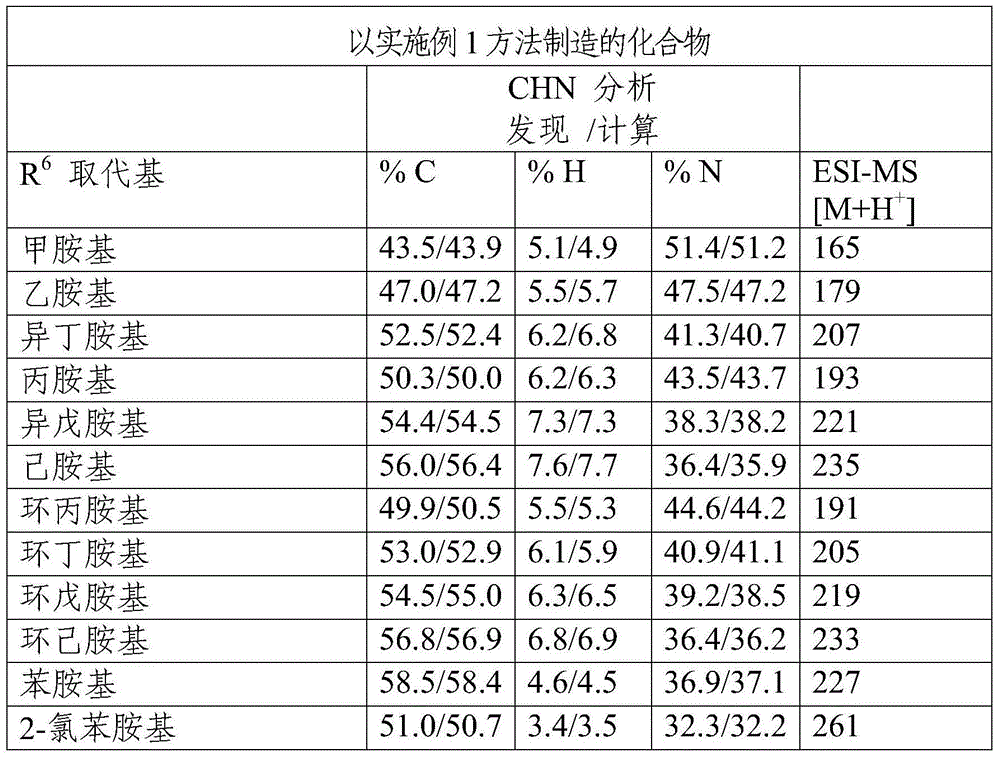
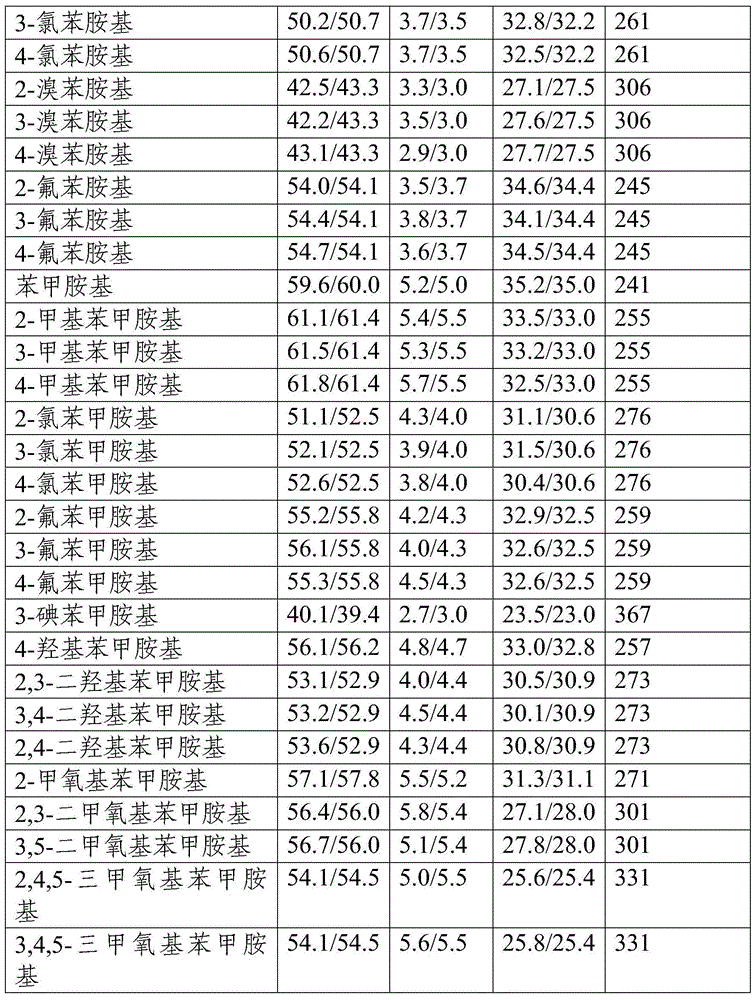
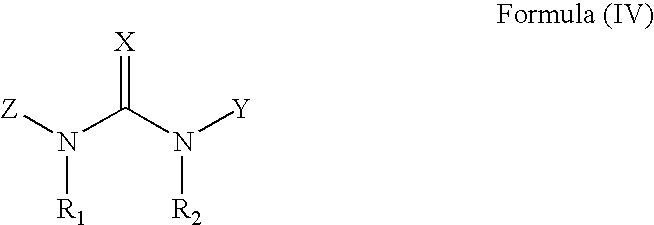Patents
Literature
85 results about "Pre diabetes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diabetes-associated markers and methods of use thereof
Owner:TETHYS BIOSCI
Method for determining insulin sensitivity with biomarkers
ActiveUS8187830B2Metabolism disorderMicrobiological testing/measurementPre diabetesBiomarker (petroleum)
Biomarkers relating to insulin resistance, pre-diabetes, type-2 diabetes, metabolic syndrome, atherosclerosis, and cardiomyopathy are provided, as well as methods for using such biomarkers as biomarkers for insulin resistance, pre-diabetes, type-2 diabetes, metabolic syndrome, atherosclerosis, and cardiomyopathy. In addition, methods for modulating the respective disorders or conditions of a subject are also provided. Also provided are suites of small molecule entities as biomarkers for insulin resistance, pre-diabetes, type-2 diabetes, metabolic syndrome, atherosclerosis, and cardiomyopathy.
Owner:METABOLON
Biomarkers for pre-diabetes, cardiovascular diseases, and other metabolic-syndrome related disorders and methods using the same
ActiveUS20090155826A1Metabolism disorderMicrobiological testing/measurementPre diabetesBiomarker (petroleum)
Biomarkers relating to insulin resistance, pre-diabetes, type-2 diabetes, metabolic syndrome, atherosclerosis, and cardiomyopathy are provided, as well as methods for using such biomarkers as biomarkers for insulin resistance, pre-diabetes, type-2 diabetes, metabolic syndrome, atherosclerosis, and cardiomyopathy. In addition, methods for modulating the respective disorders or conditions of a subject are also provided. Also provided are suites of small molecule entities as biomarkers for insulin resistance, pre-diabetes, type-2 diabetes, metabolic syndrome, atherosclerosis, and cardiomyopathy.
Owner:METABOLON
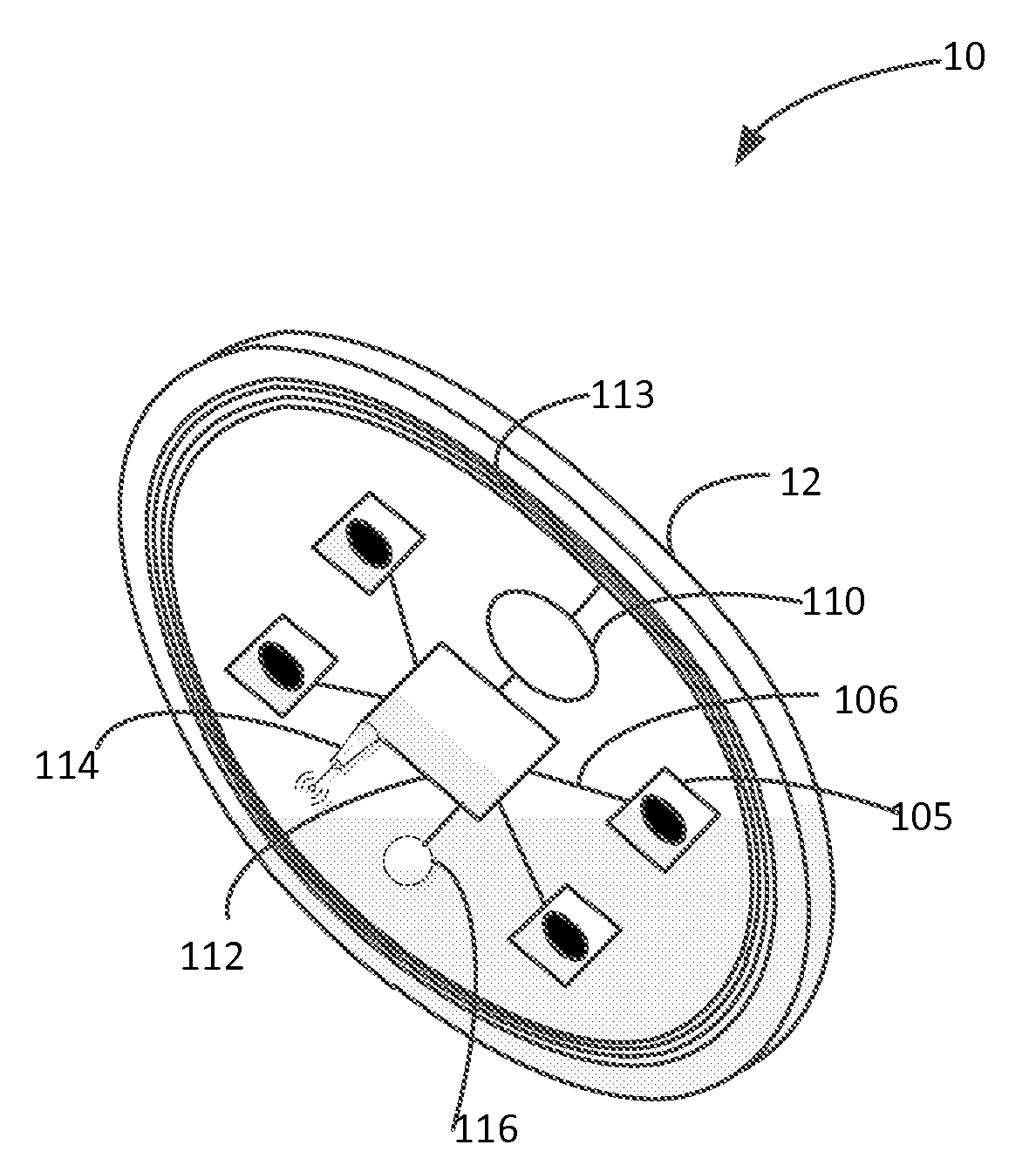
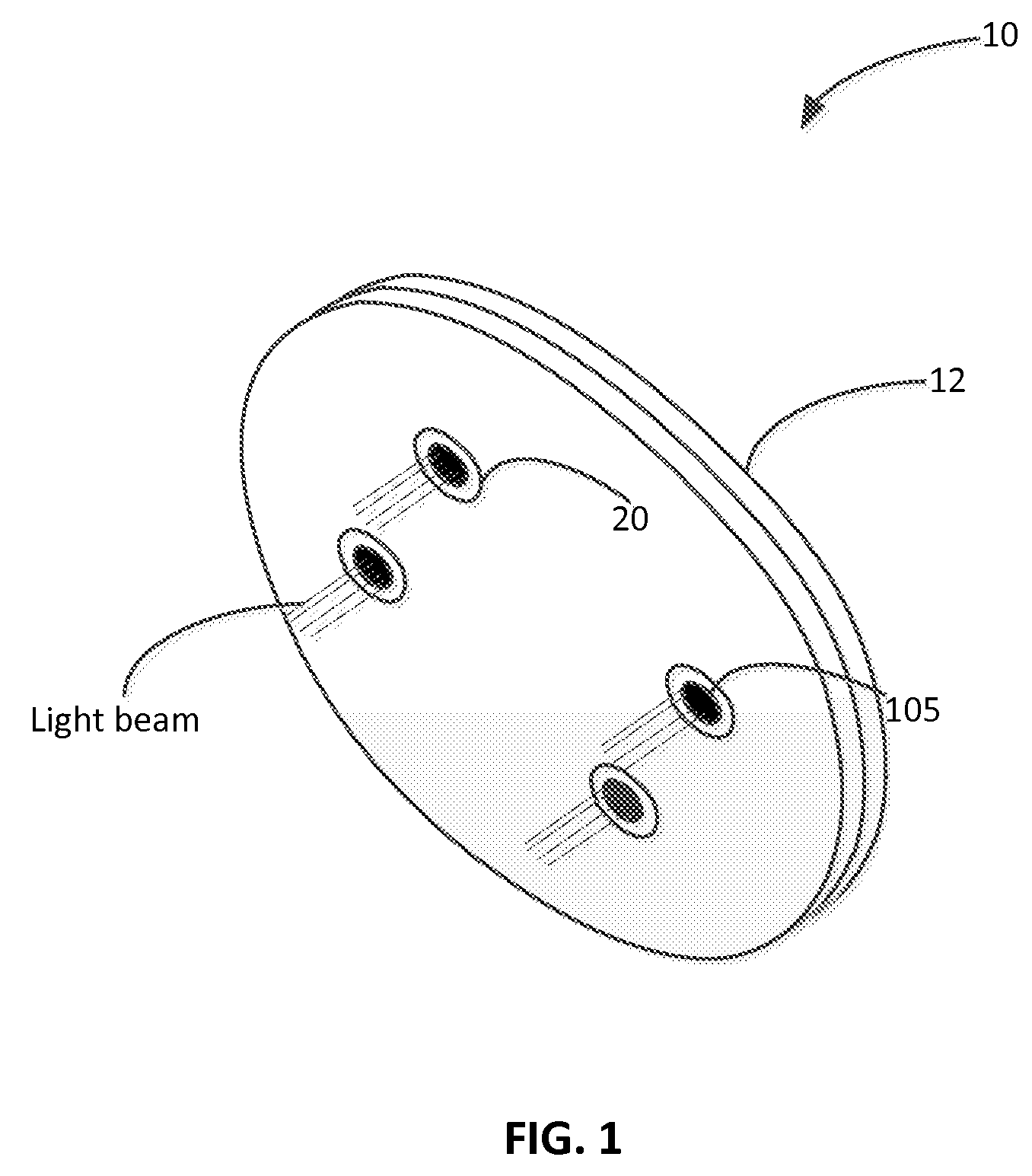
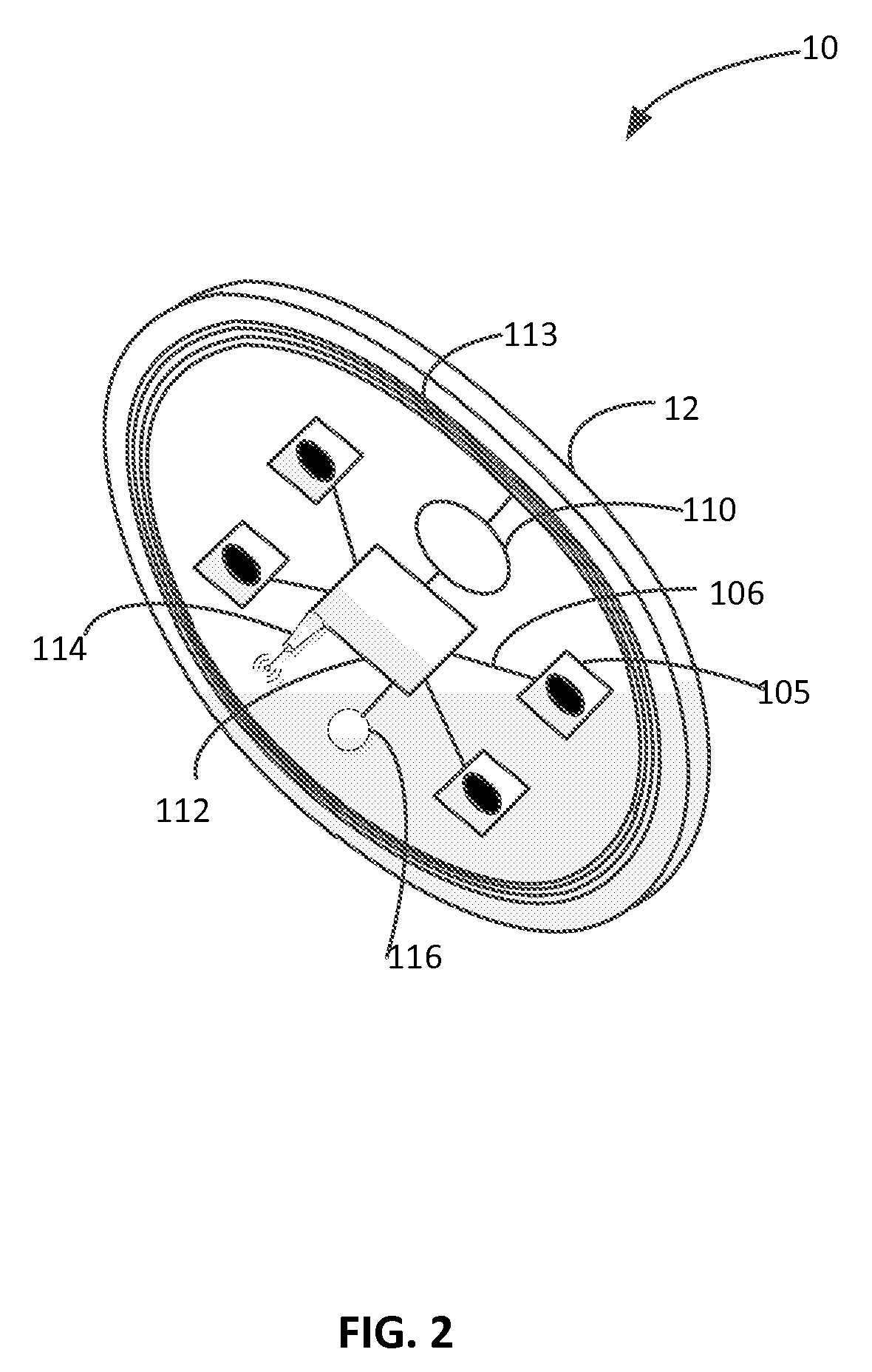
Light Therapy for Treating or Managing Diabetes and Metabolic Syndrome
Techniques for managing diabetes and pre-diabetes using light are disclosed herein. In one example, a light generating device is positioned to a body part of a diabetic or pre-diabetic patient, and a beam of light generated by the light generating device is directed to the body part of the patient to control blood sugar level of the patient. In various embodiments, the beam of light directed to the patient can also help to control the blood lipid level such as triglyceride level, blood liver enzyme of the patient, and various other symptoms of diabetes and pre-diabetes. In various embodiments, the body part includes body area rich in adipose tissue, such as the abdominal area, thigh, buttocks, and upper arms of the patient.
Owner:PRESCOTT MARVIN A
Metabolic Disease Treatments
The invention relates to the use of compounds to treat a number of conditions, such as a pre-diabetes condition, type 1 diabetes, type 2 diabetes, hyperglycemia, insulin resistance and glucose intolerance. Compounds that can be used in one or more of the treatment methods include 3β,7β,16α,17β-tetrahydroxyandrost-5-ene, 3α,7β,16α,17β-tetrahydroxyandrost-5-ene, 3β,7β,16α,17β-tetrahydroxyandrost-5-ene, 3β,16α,17β-trihydroxyandrost-5-ene-7-one, 3β,7β,17β-trihydroxy-17α-ethynylandrost-5-ene, 3β,17β-dihydroxy-17α-ethynylandrost-5-ene-7-one and 3β,7α,17β-trihydroxy-17α-ethynylandrost-5-ene.
Owner:HARBOR DIVERSIFIED +2
Therapeutic process for the treatment of the metabolic syndrome and associated metabolic disorders
Owner:VEROSCI
Pharmaceutical compositions and methods for metabolic modulation
InactiveUS20070161582A1Improved pharmaceutical compositionBiocideMetabolism disorderPre diabetesCytokinin
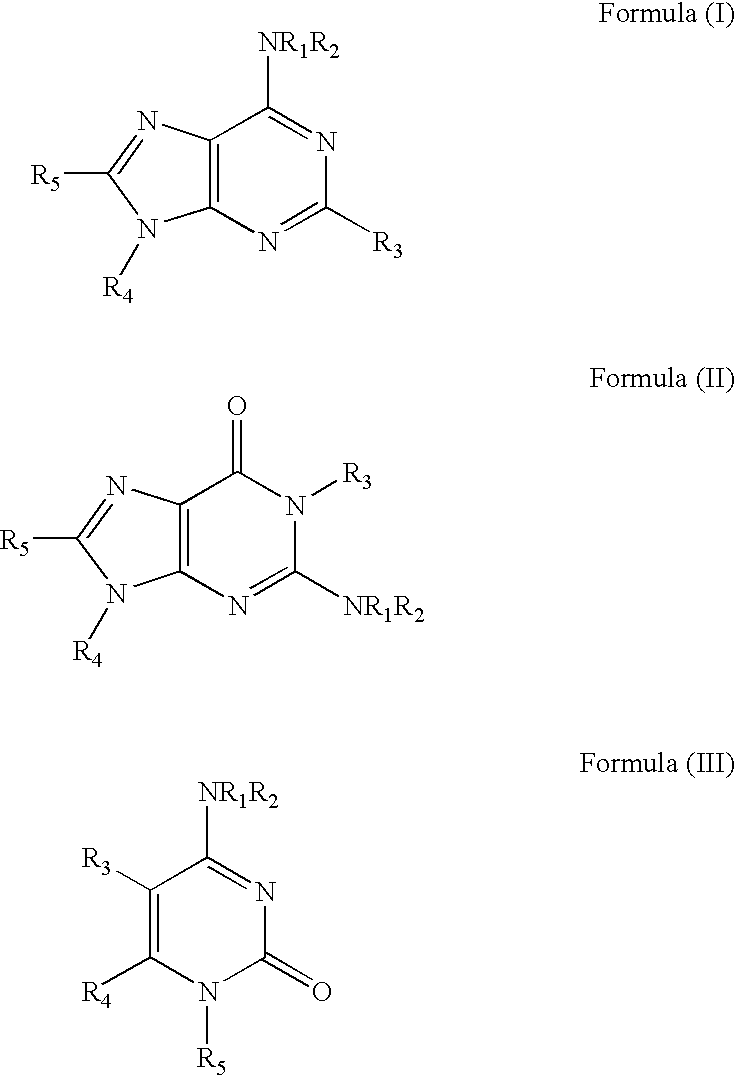
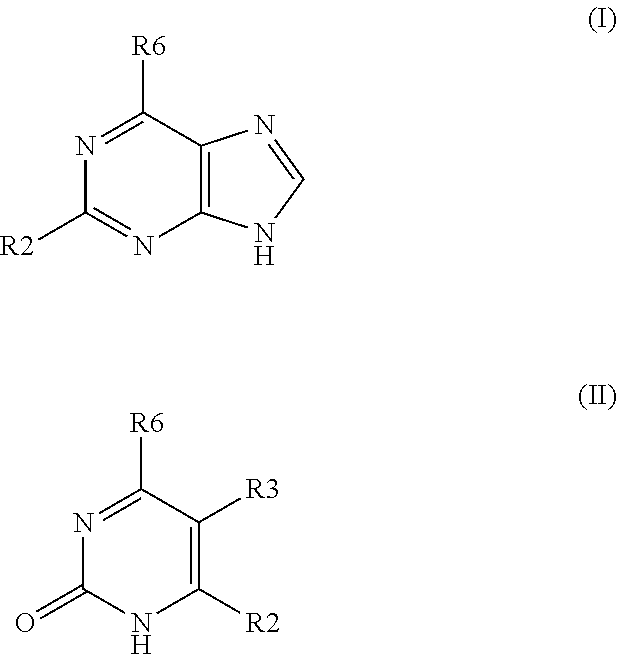
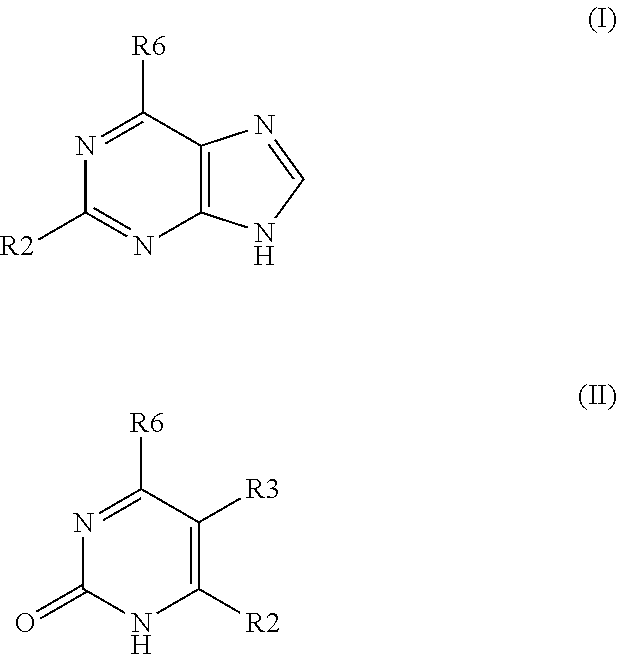
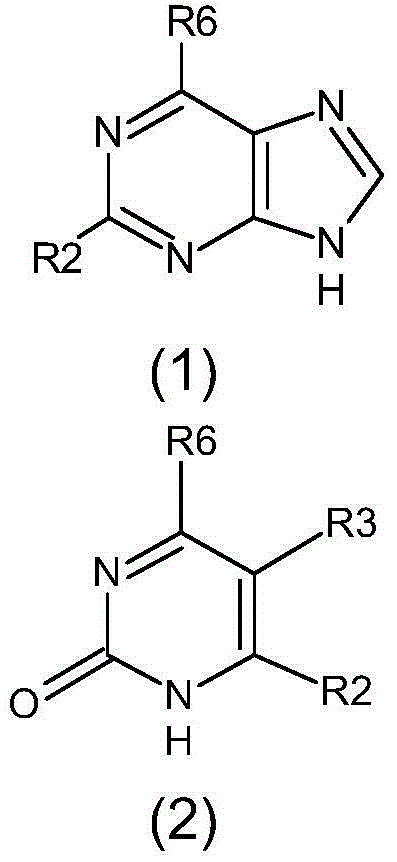
Pharmaceutical compositions include compounds with cytokinin activity to modulate glucose and / or lipid metabolism in a mammal. Especially preferred compounds include those comprising a purine scaffold, and it is further preferred that contemplated compositions are employed to prevent and / or treat various diseases, including pre-diabetes, insulin resistance, type-2 diabetes, Syndrome X, and dyslipidemia In still further preferred aspects, compounds with cytokinin activity are used to activate AMPK and / or Akt. Consequently, various diseases associated with dysregulation of AMPK and / or Akt may be treated using the compounds of the present inventive subject matter.
Owner:VDF FUTURECEUTICALS
Method for treating disease or condition susceptible to amelioration by AMPK activators and compounds of formula which are useful to activate AMP-activated protein kinase (AMPK)
The present invention relates to a method for treating disease or condition susceptible to amelioration by AMPK activators and compounds of formula which are useful to activate AMP-activated protein kinase (AMPK) and the use of the compounds in the prevention or treatment of disease, including pre-diabetes, type 2 diabetes, syndrome X, metabolic syndrome and obesity.
Owner:ENERGENESIS BIOMEDICAL
Pharmaceutical composition, methods for treating and uses thereof
ActiveUS9949998B2Good effectFew complianceOrganic active ingredientsMetabolism disorderPre diabetesKidney injury
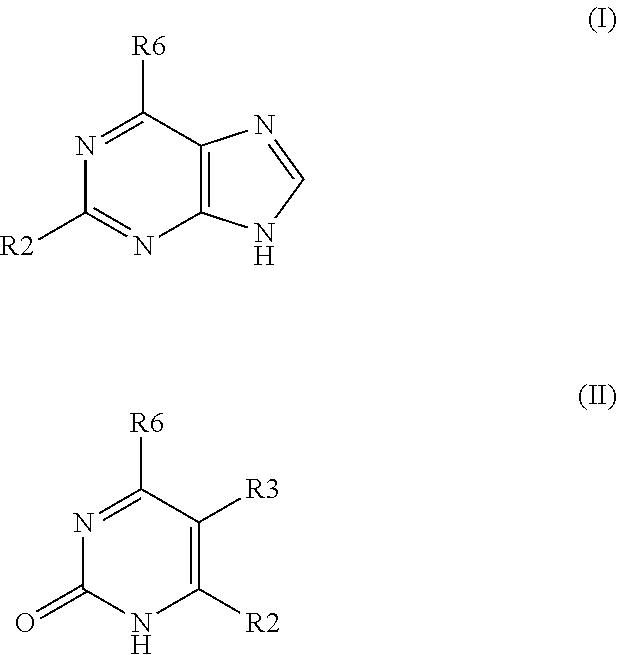
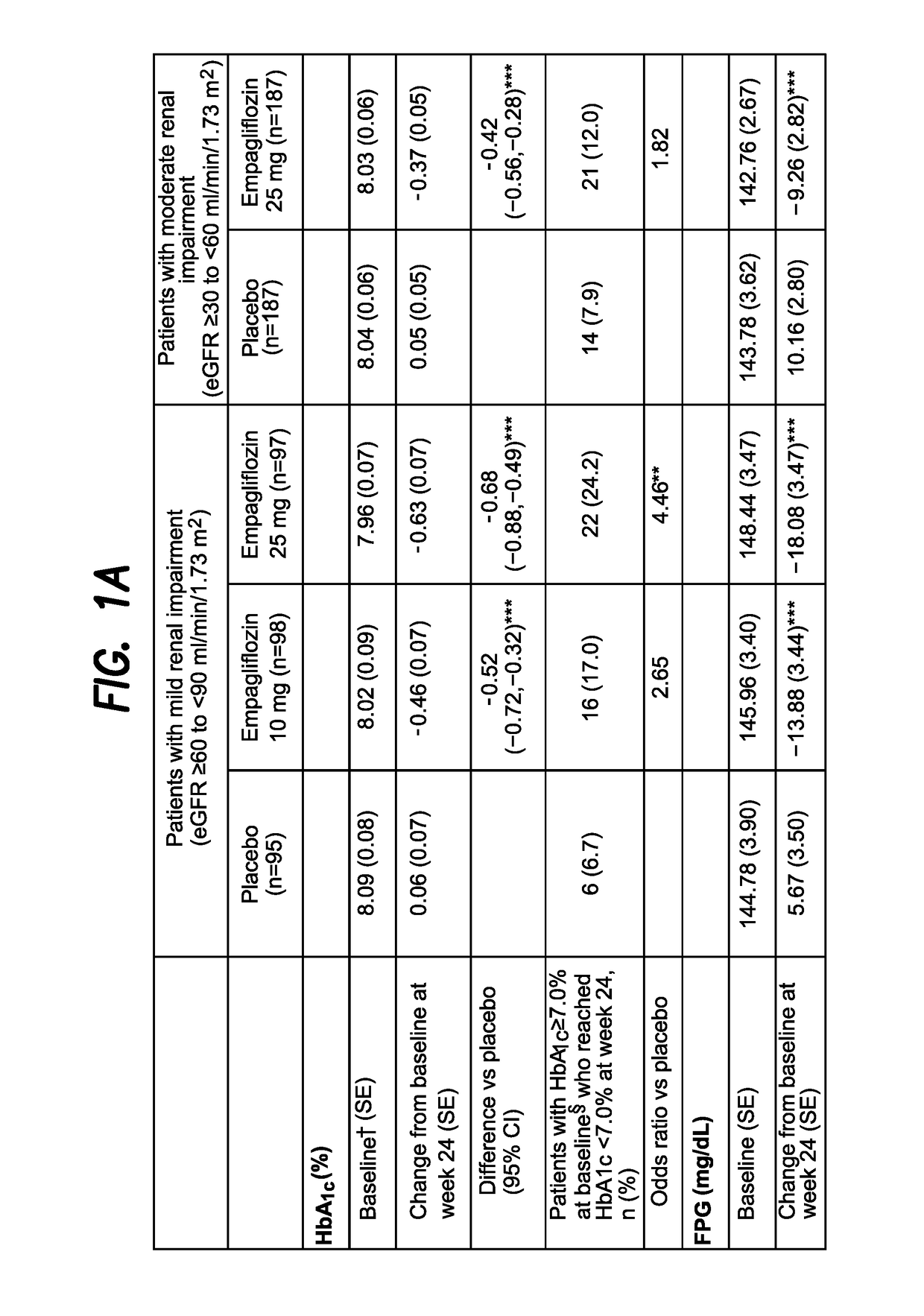
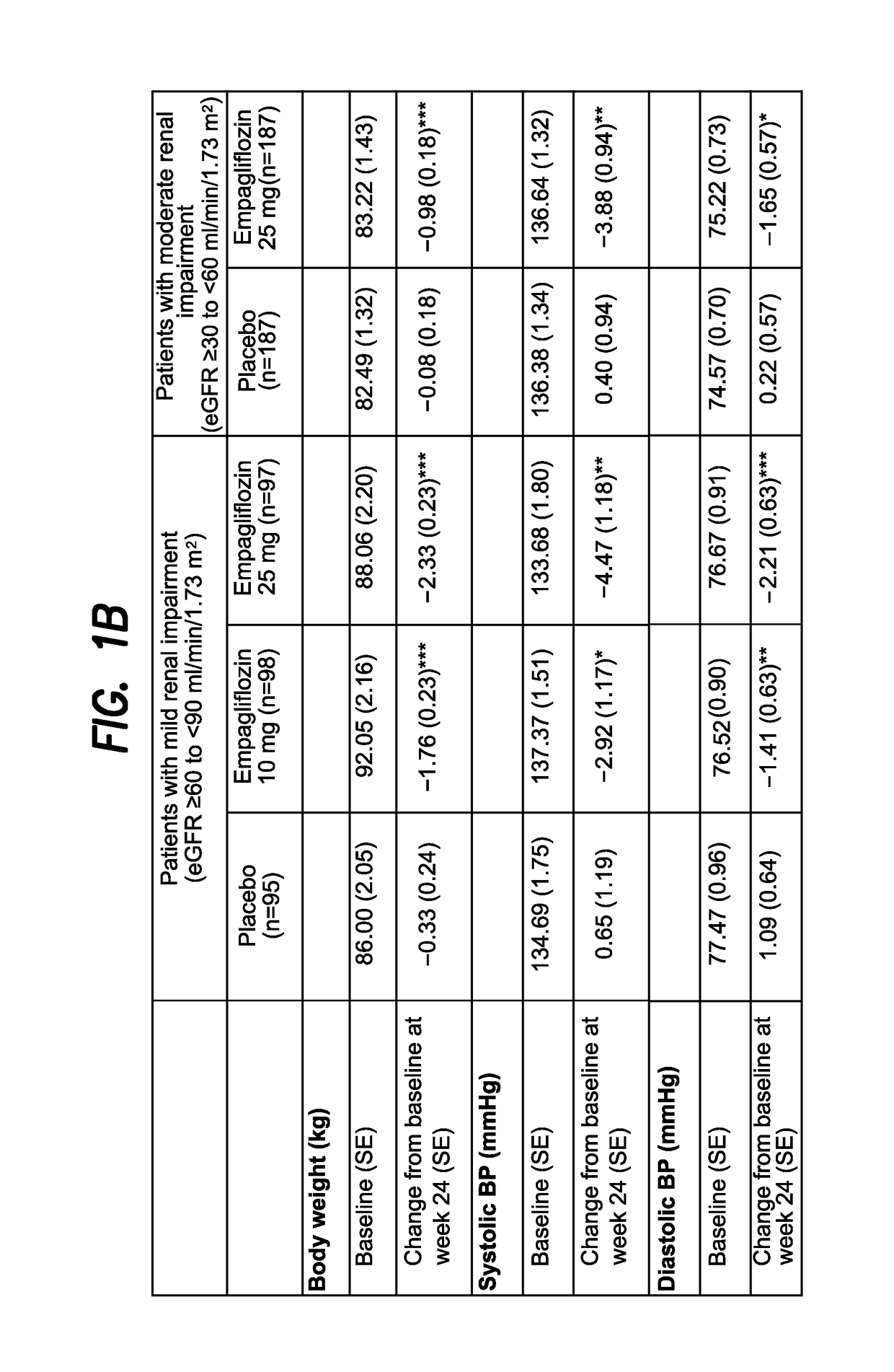
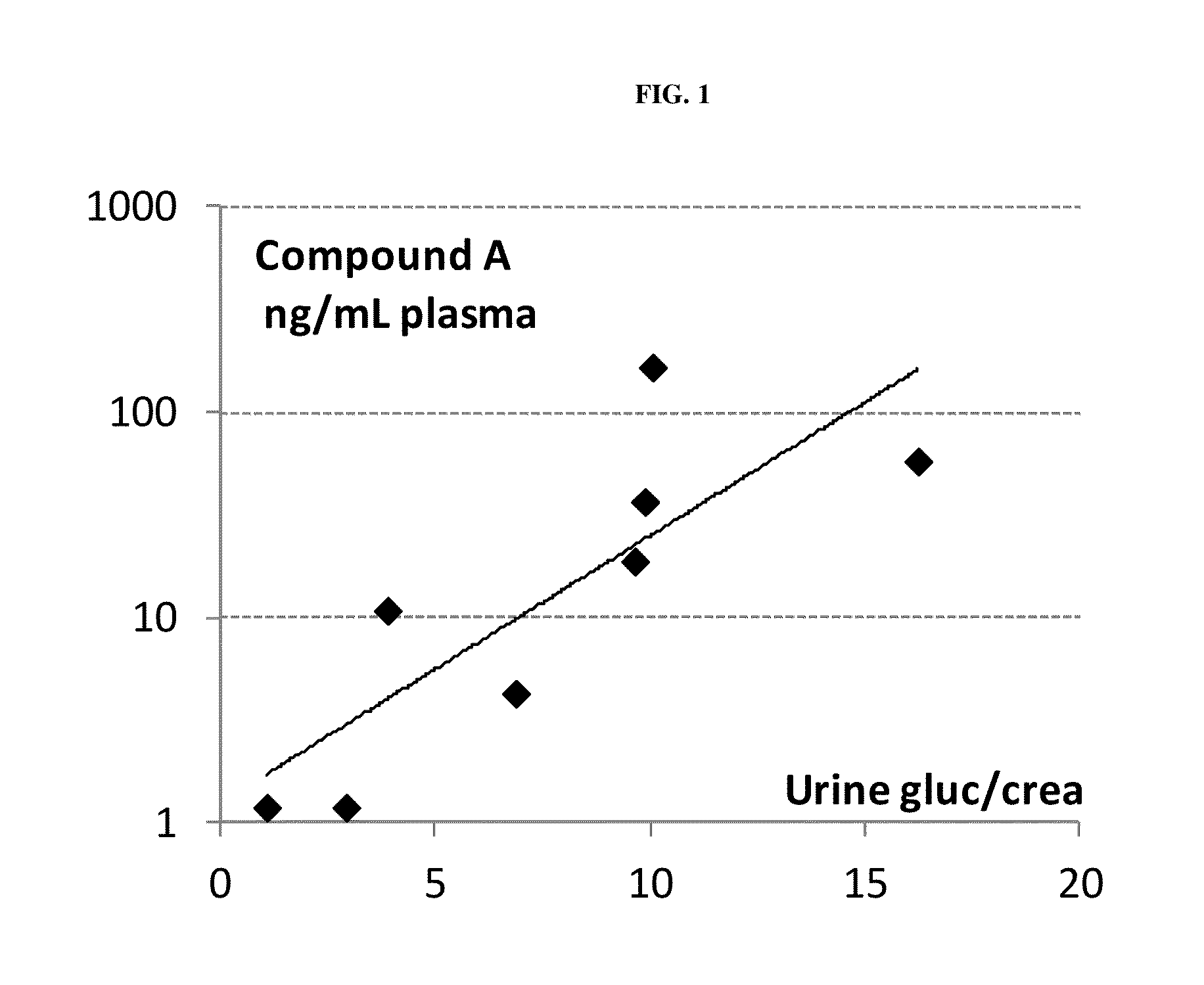
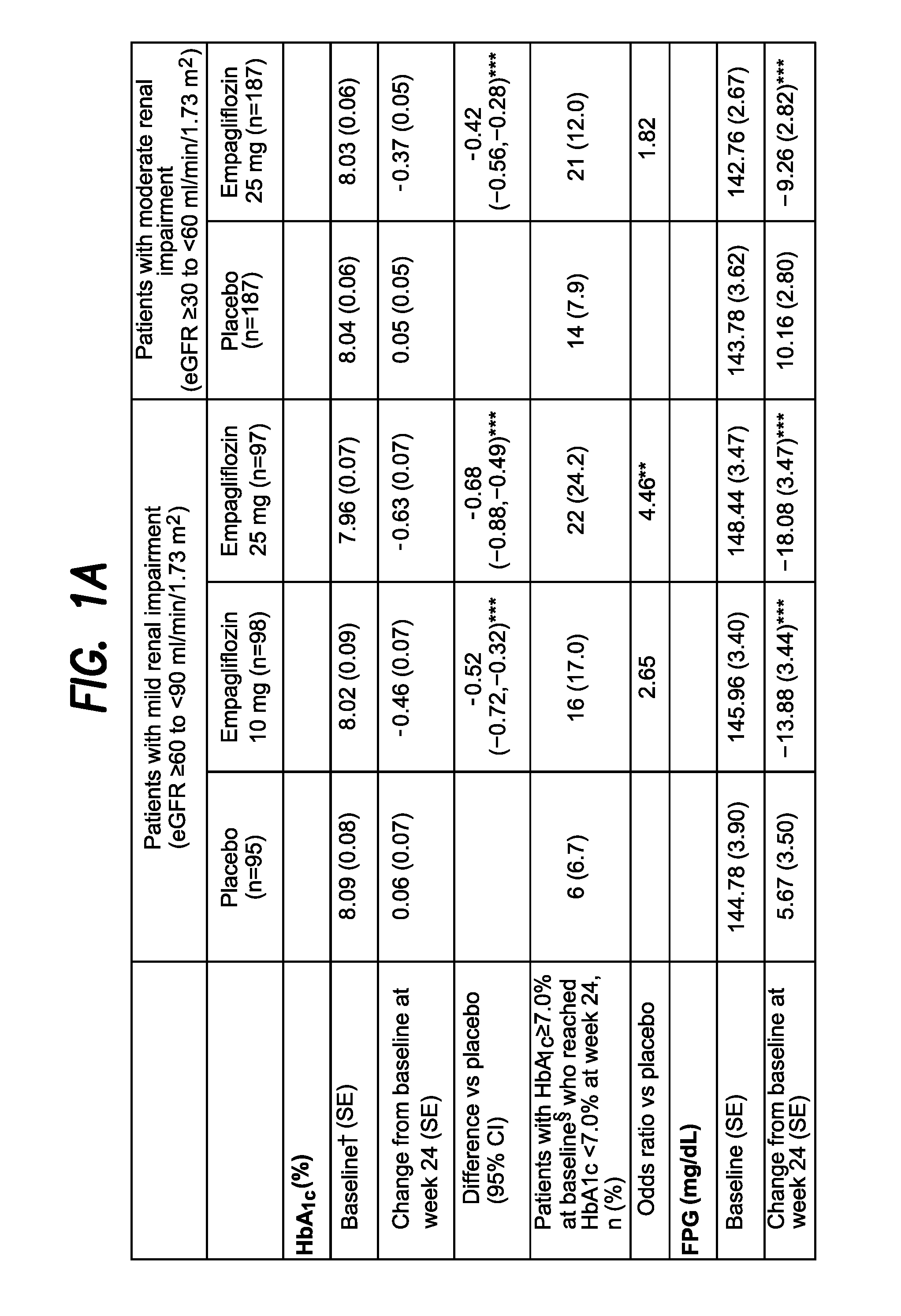
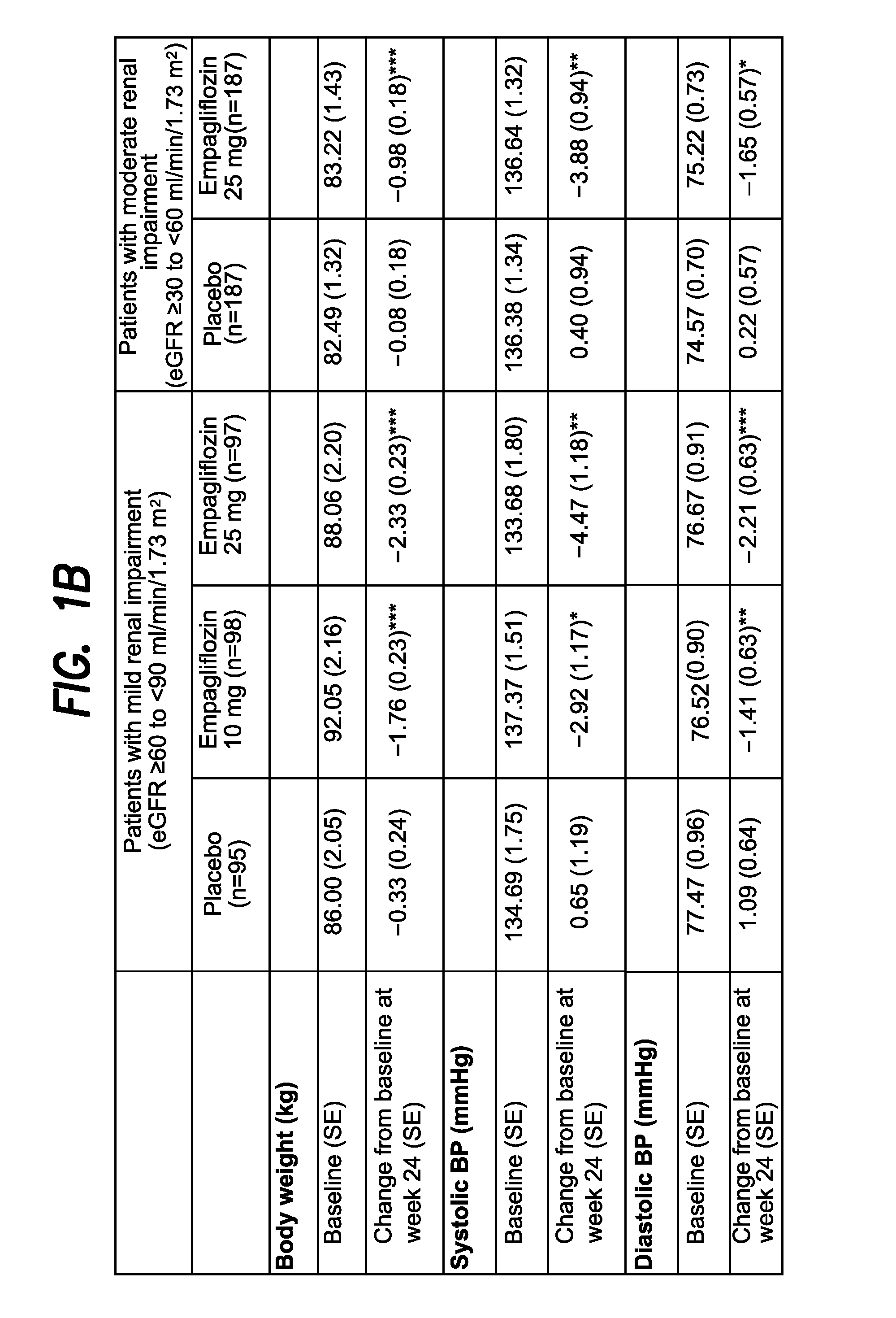
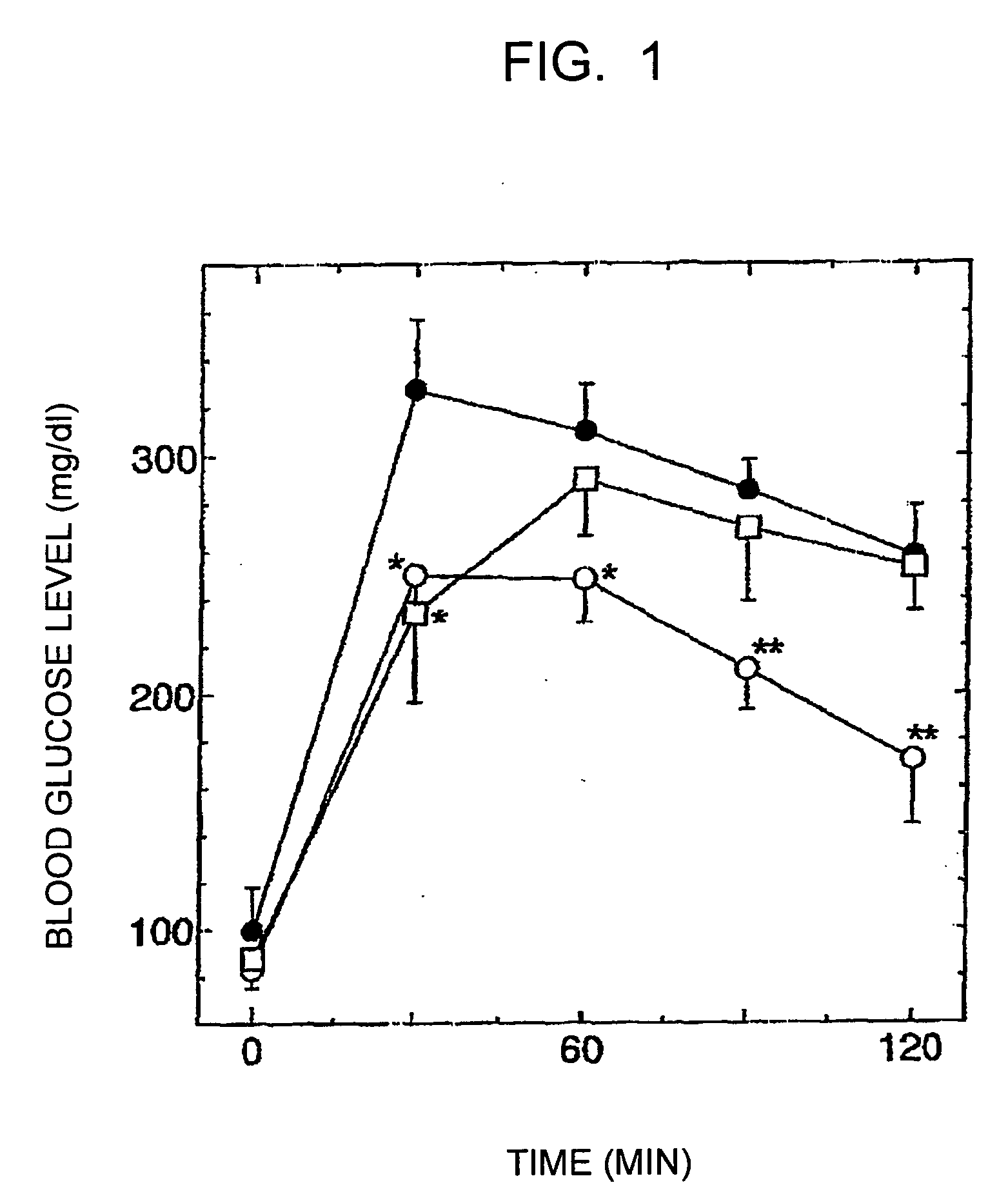
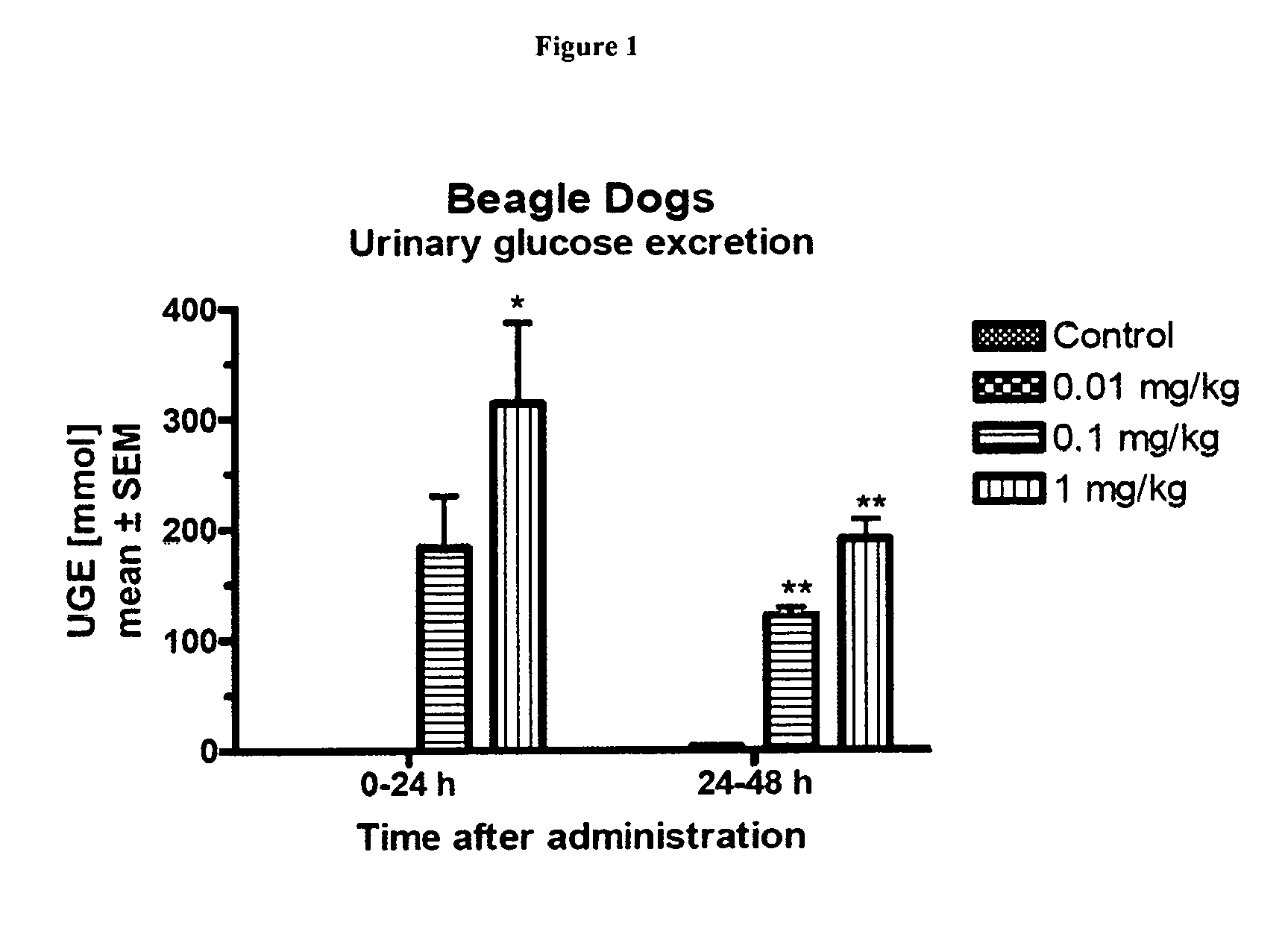
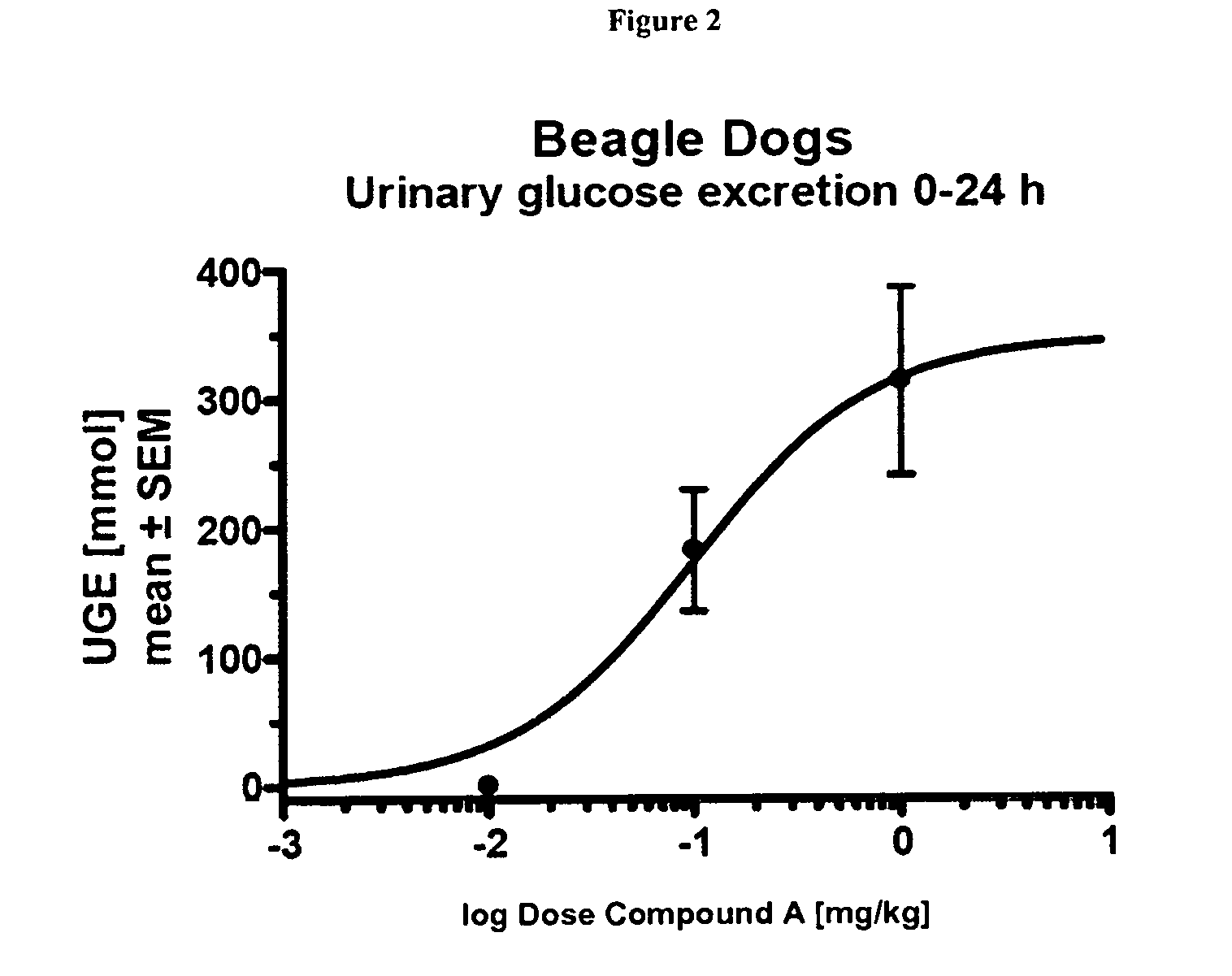
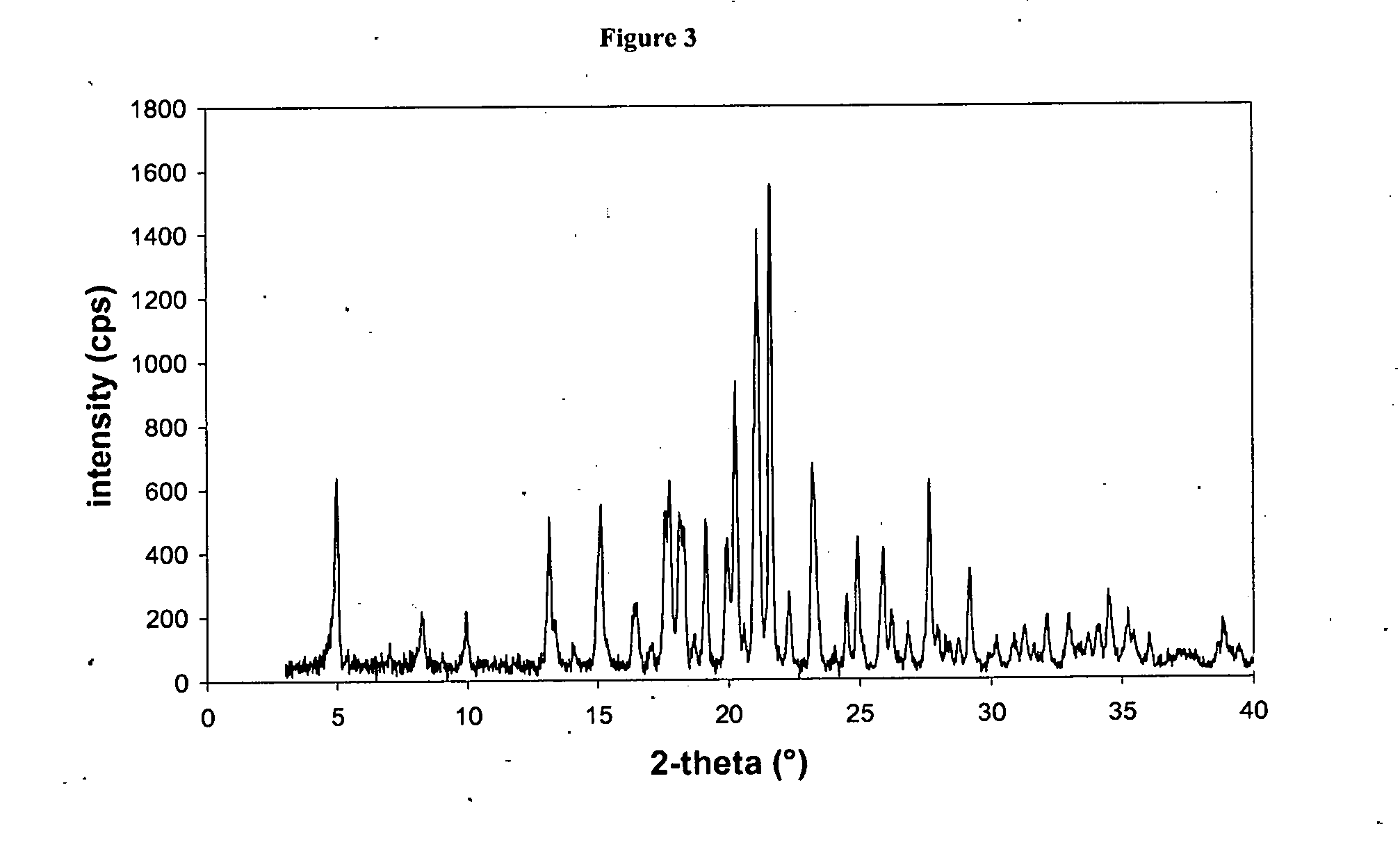
The present invention relates to certain SGLT-2 inhibitors for treating and / or preventing metabolic disorders, such as type 1 or type 2 diabetes mellitus or pre-diabetes, in patients with renal impairment or chronic kidney disease (CKD).
Owner:BOEHRINGER INGELHEIM INT GMBH
Treatment of metabolic disorders in feline animals
ActiveUS20150164856A1Reduce doseReduce frequencyBiocideNervous disorderAcute hyperglycaemiaDyslipidemia
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a feline animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, diabetes mellitus type 1 or type 2, insulin resistance, obesity, hyperglycemia, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, atherosclerosis, inflammation of the pancreas, neuropathy and / or Syndrome X (metabolic syndrome) and / or loss of pancreatic beta cell function and / or wherein the remission of the metabolic disorder, preferably diabetic remission, is achieved and / or maintained.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
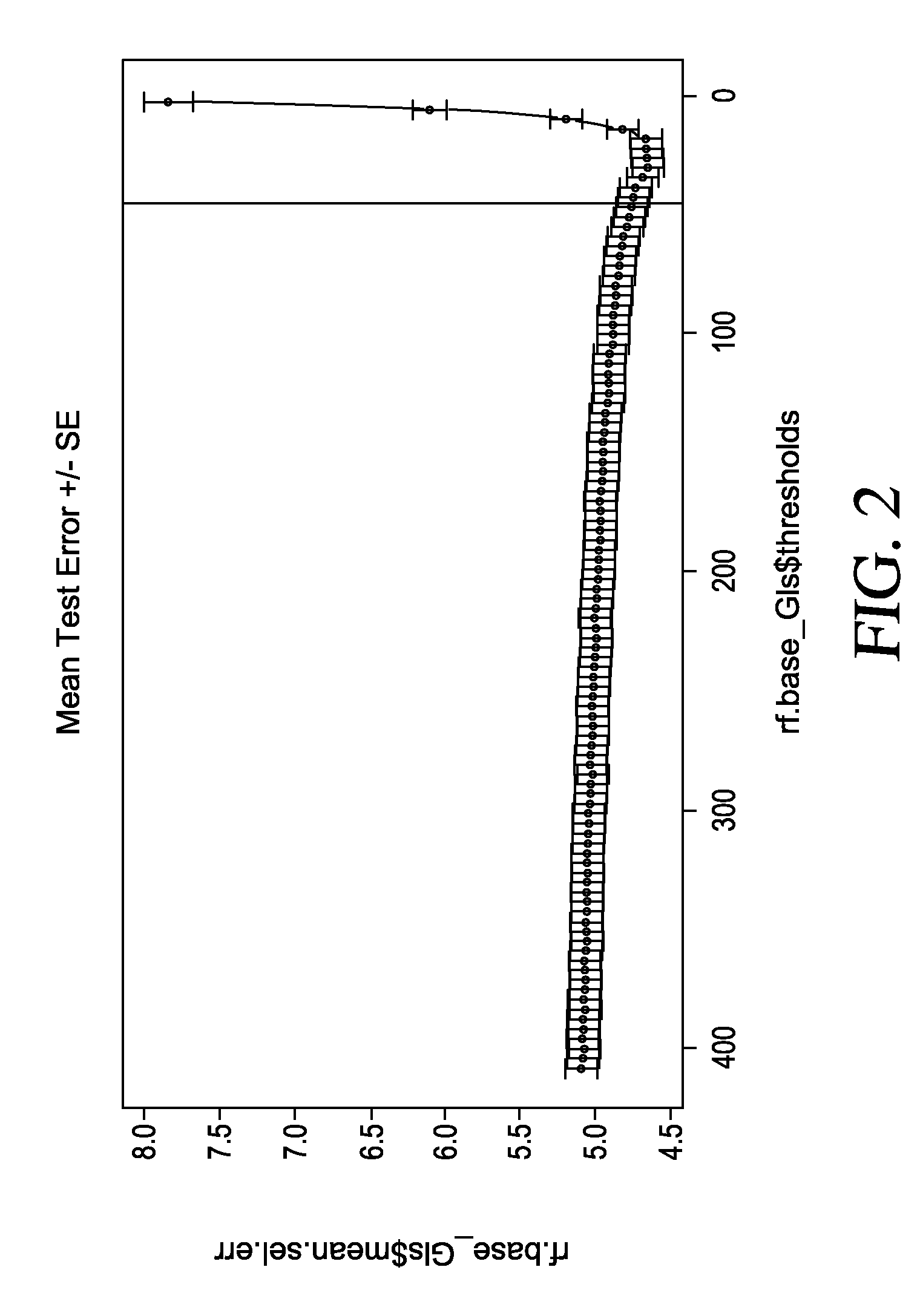
System, Method and Computer Simulation Environment For In Silico Trials in Pre-Diabetes and Type 2 Diabetes
ActiveUS20120130698A1Provide informationIneffective treatmentMedical simulationMedical data miningGlucose utilizationModel dynamics
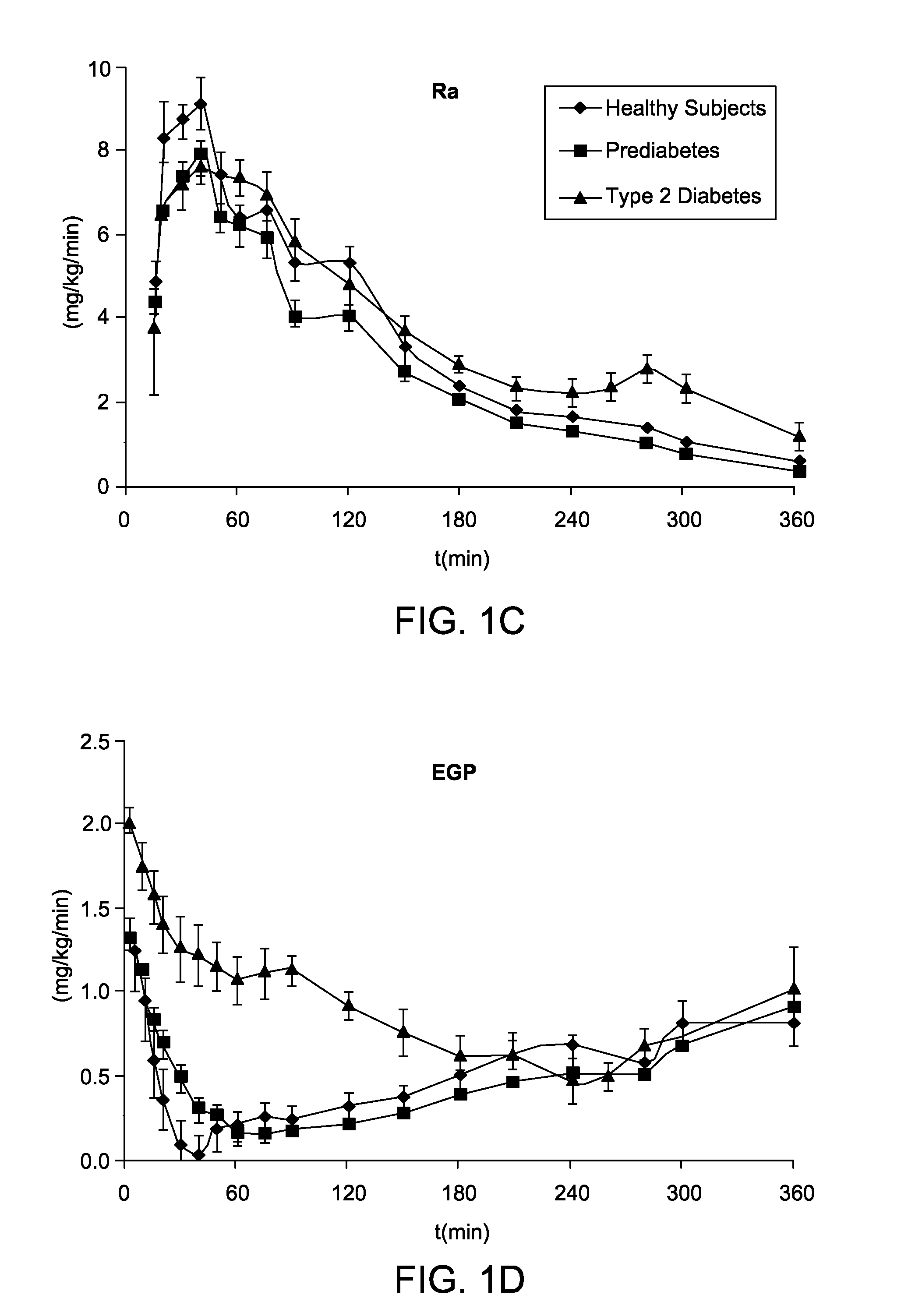
An electronic system is provided that simulates a glucose-insulin metabolic system of a T2DM or prediabetic subject, wherein the system includes a subsystem that models dynamic glucose concentration in a T2DM or prediabetic subject, including an electronic module that models endogenous glucose production (EGP(t)), or meal glucose rate of appearance (Ra(t>>, or glucose utilization (U(t)), or renal excretion of glucose (B(t)), a subsystem that models dynamic insulin concentration in said T2DM or prediabetic subject, including an electronic module that models insulin secretion (S(t)), an electronic database containing a population of virtual T2DM or prediabetic subjects, each virtual subject having a plurality of metabolic parameters, and a processing module that calculates an effect of variation of at least one metabolic parameter value on the glucose insulin metabolic system of a virtual subject by inputting the plurality of metabolic parameter values.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Analogs of 4-hydroxyisoleucine and uses thereof
The invention relates to analogs of 4-hydroxyisoleucine, and to lactones, pharmaceutically acceptable salts, and prodrugs thereof, to processes for their preparation, and to pharmaceutical compositions comprising the same. The analogs of the invention stimulate both glucose uptake and insulin secretion, and may thus be useful for the prevention and treatment of disorders of carbohydrate or lipid metabolism, including diabetes mellitus (type 1 and type 2 diabetes), pre-diabetes, and Metabolic Syndrome.
Owner:INNODIA INC +2
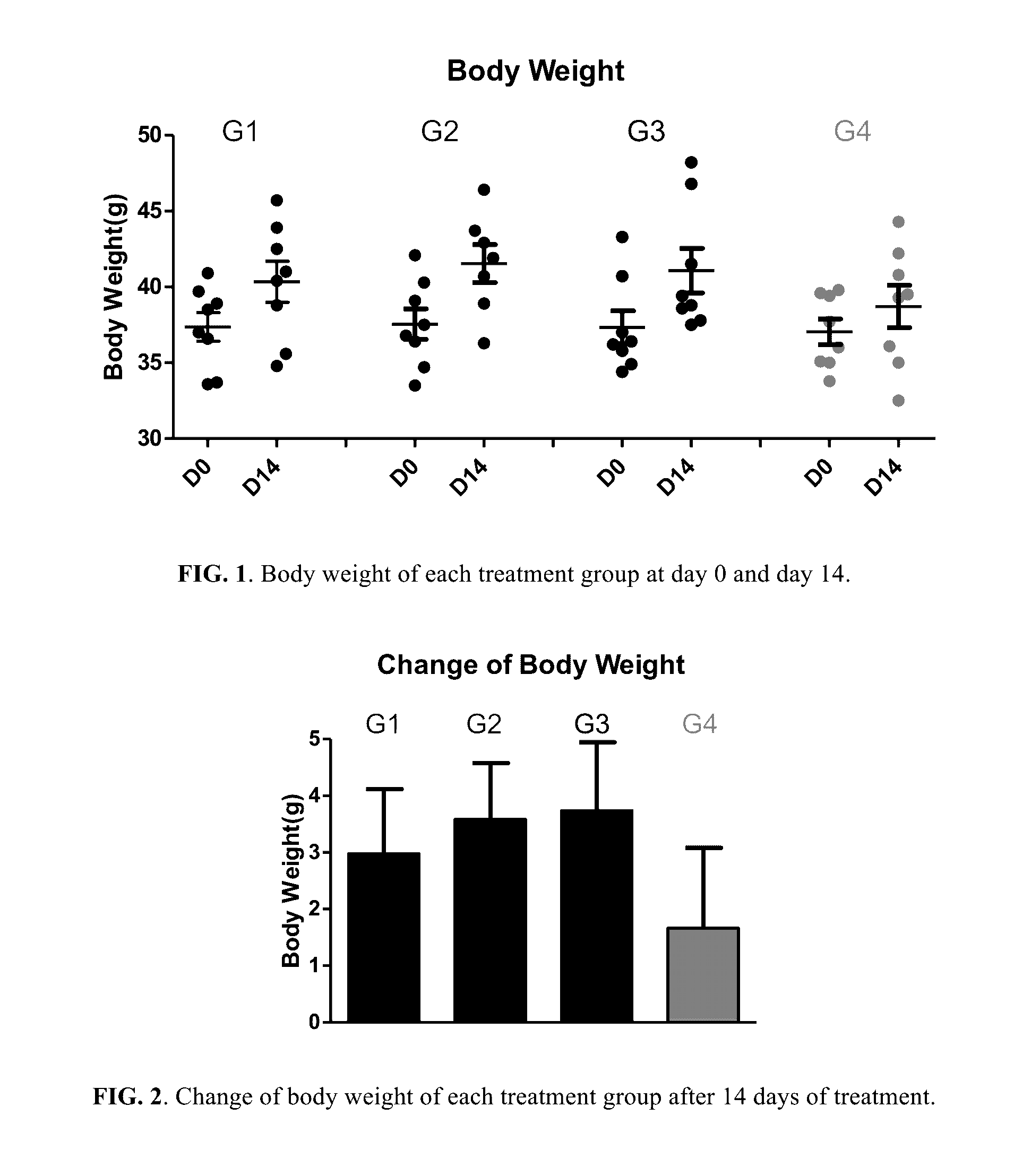
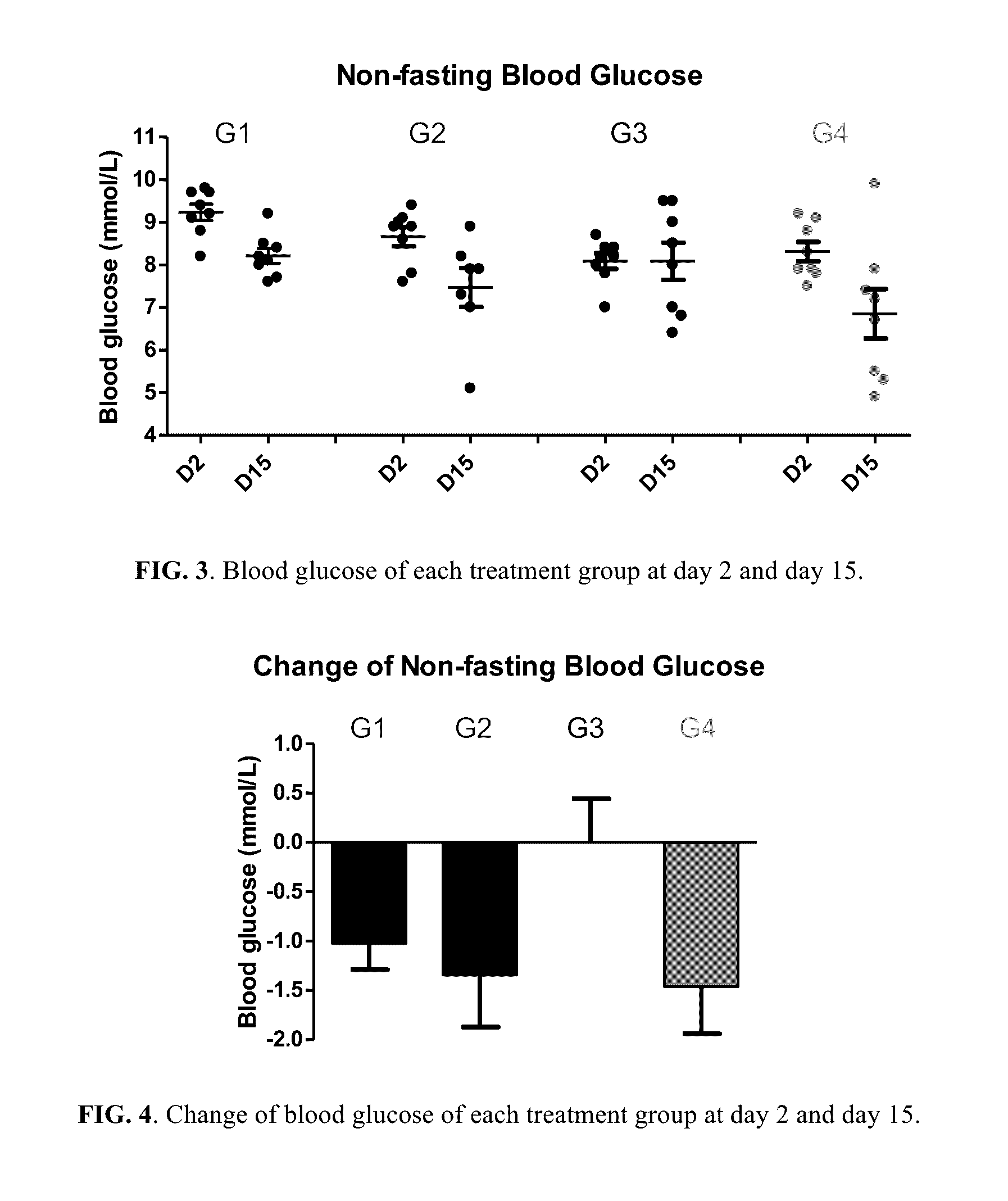
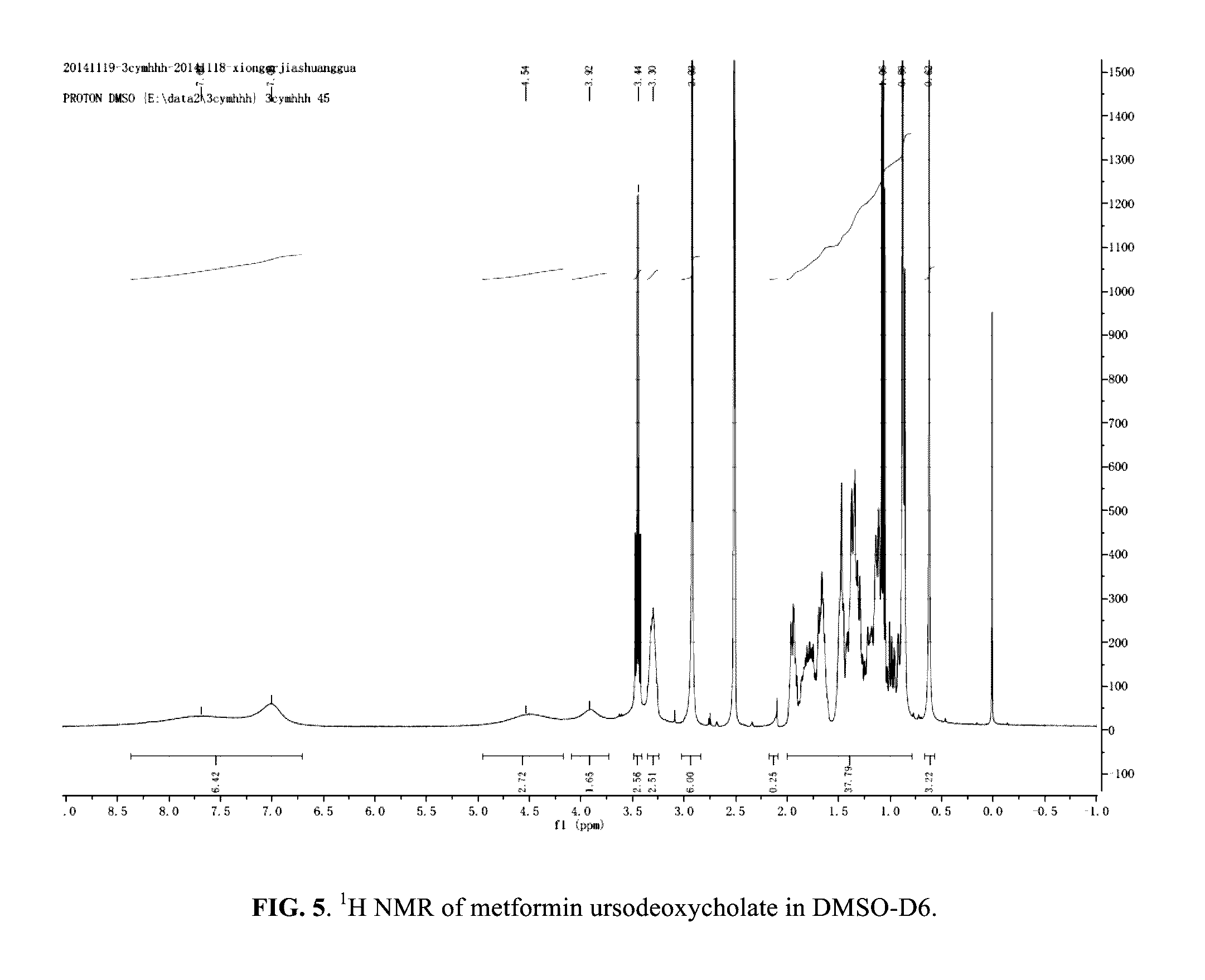
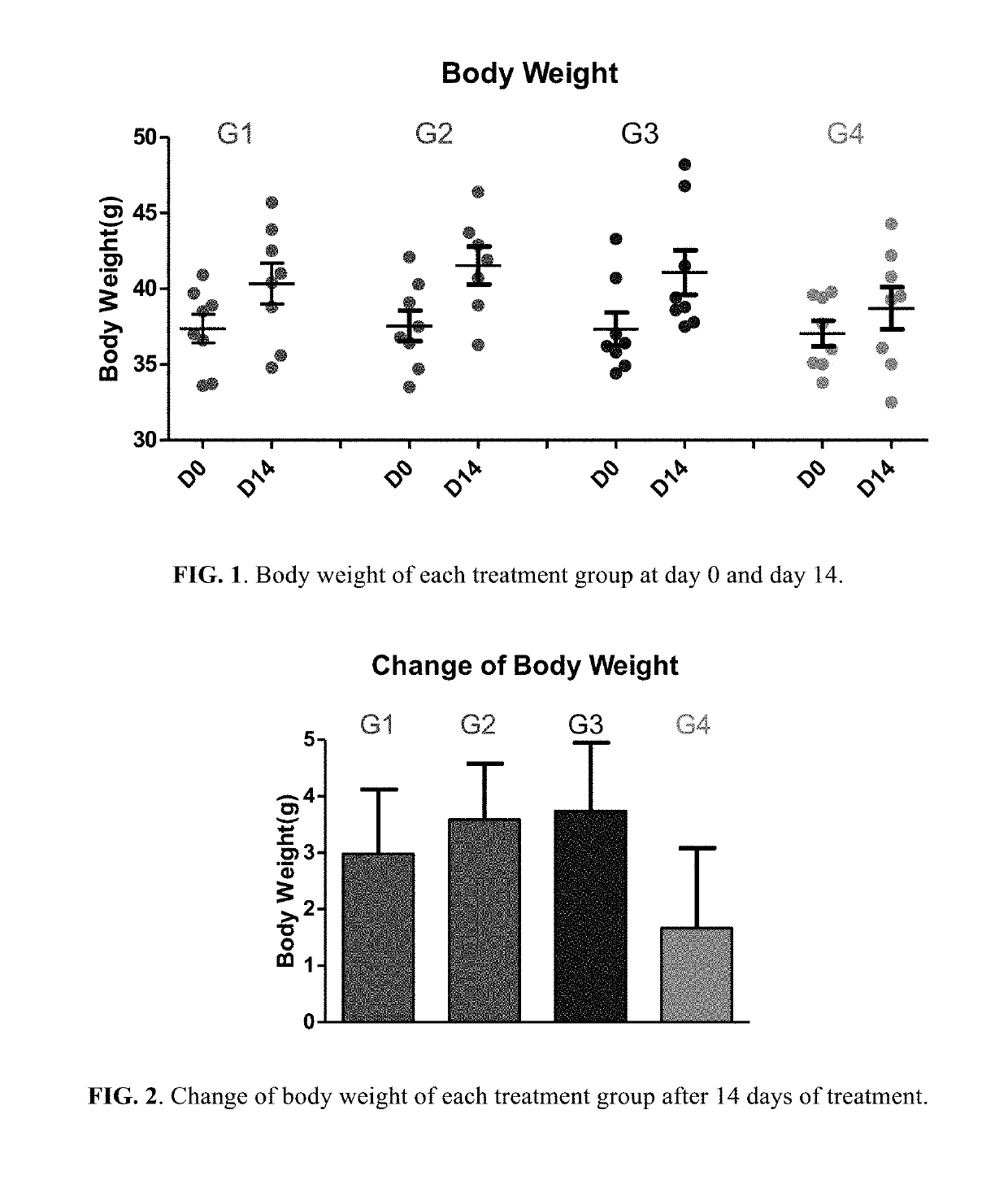
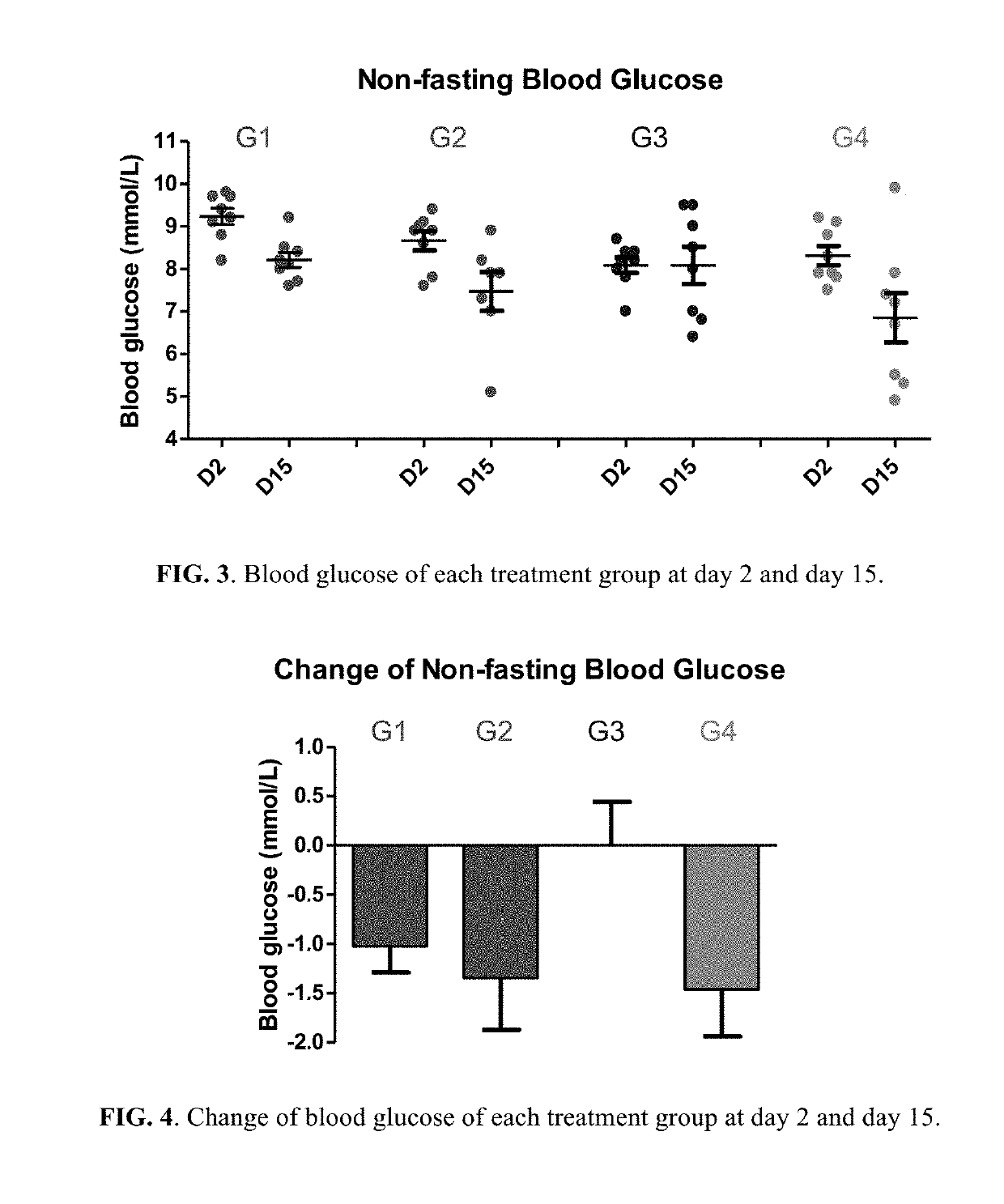
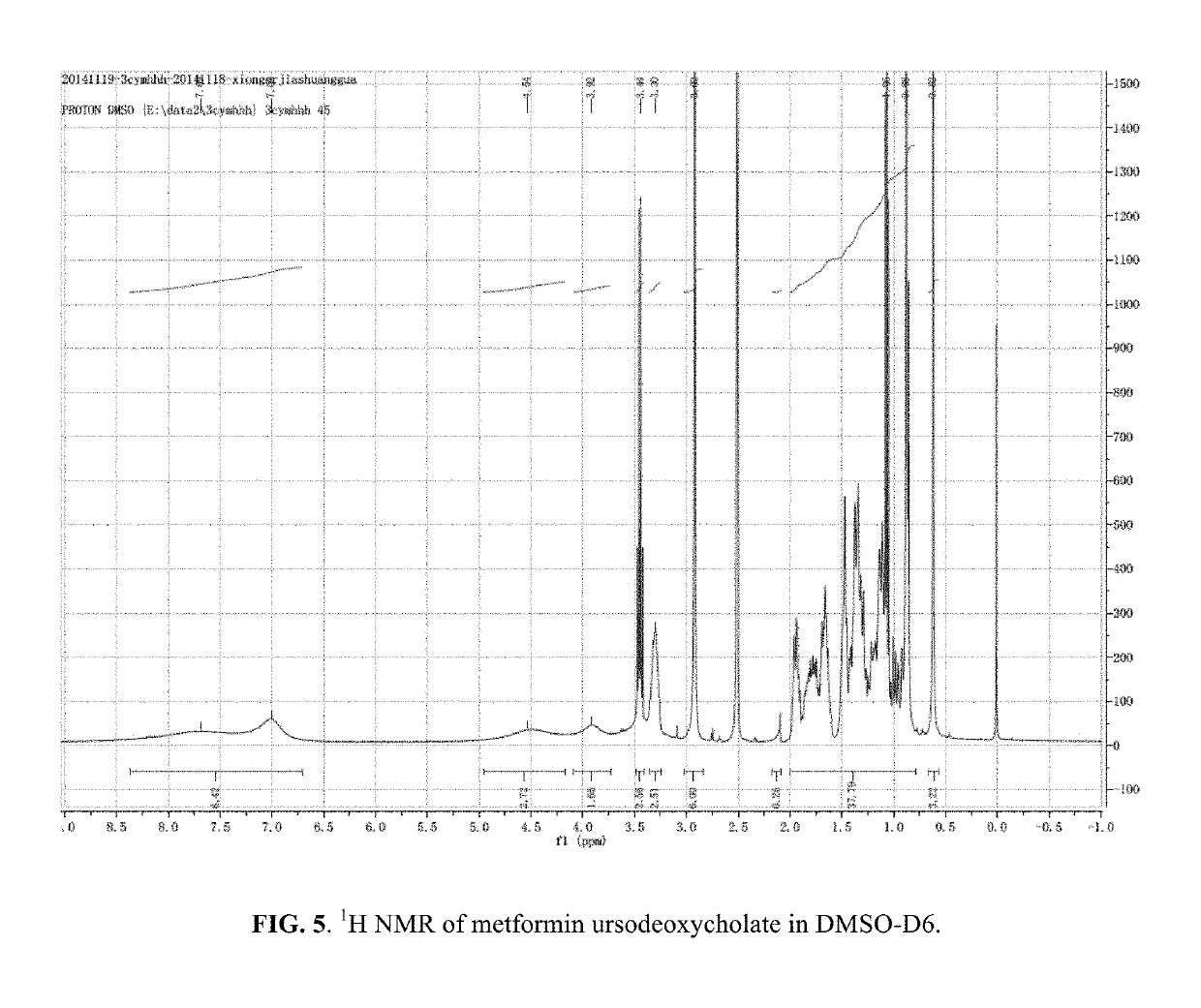
Berberine salts, ursodeoxycholic salts and combinations, methods of preparation and application thereof
Owner:SHENZHEN HIGHTIDE BIOPHARM
Berberine salts, ursodeoxycholic salts and combinations, methods of preparation and application thereof
Owner:SHENZHEN HIGHTIDE BIOPHARM
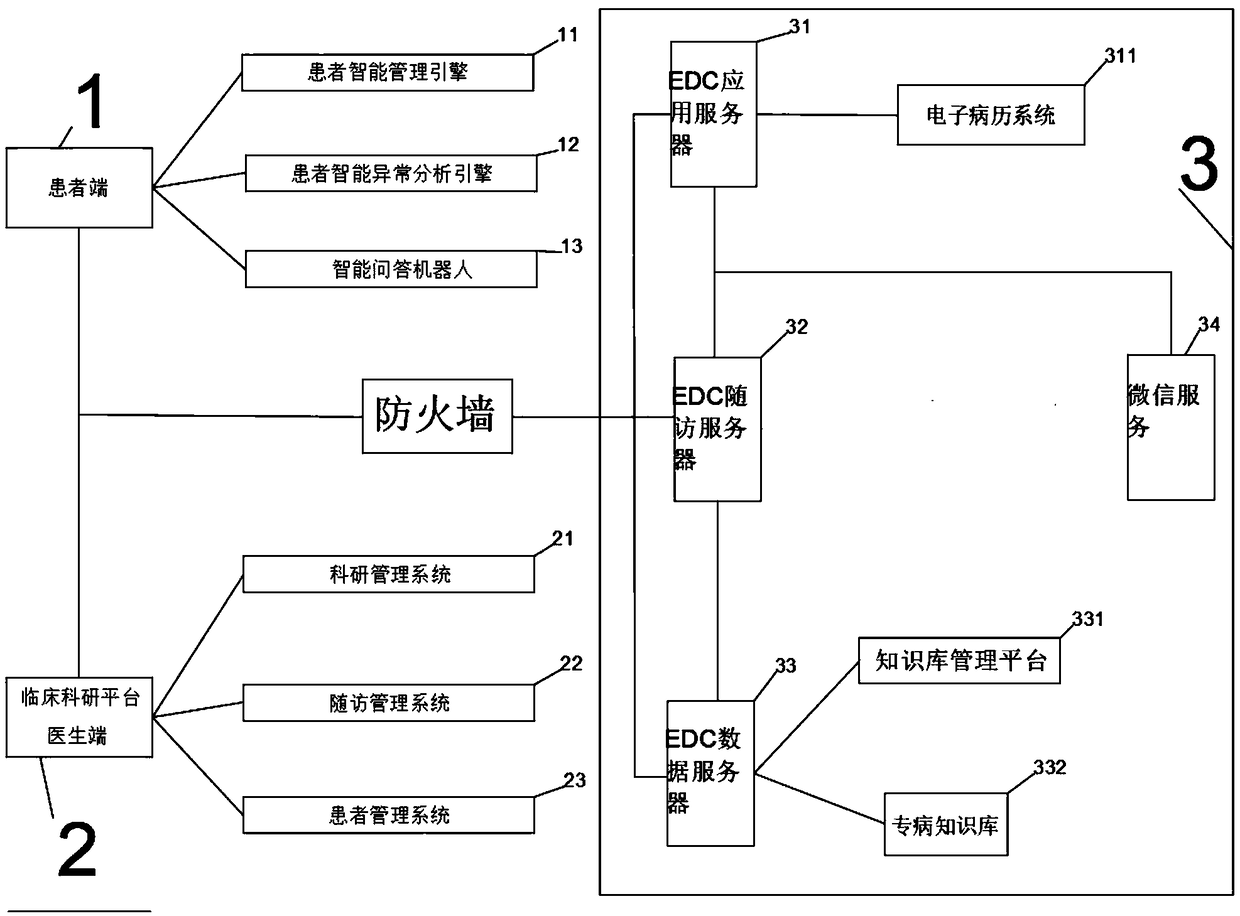
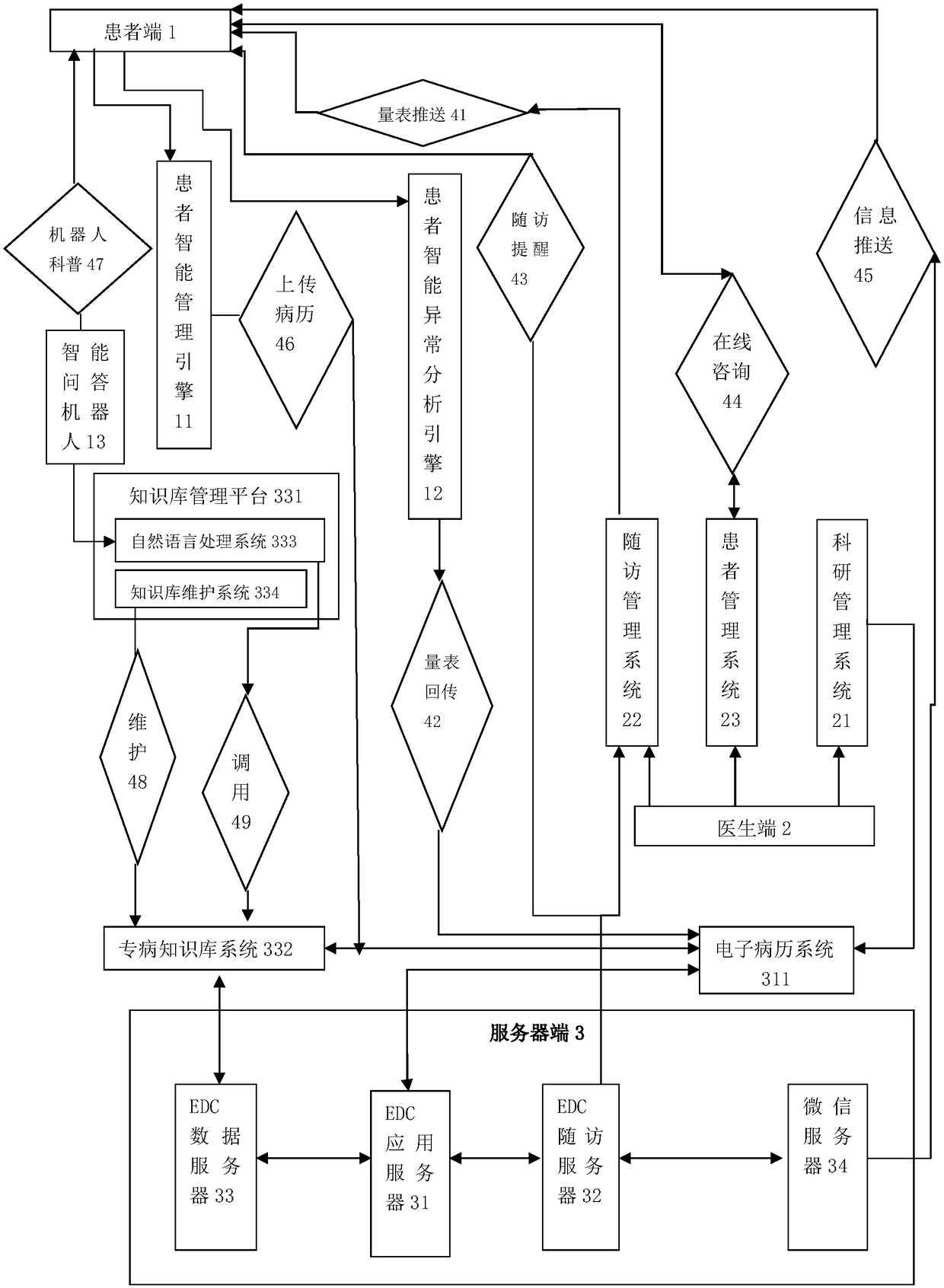
Pre-diabetes PC-WeChat intelligent management and follow-up system
InactiveCN109102847ARealize automatic collectionRealize instant queryMedical equipmentPatient-specific dataMedical recordDisease
The invention relates to a pre-diabetes PC-WeChat intelligent management and follow-up system. The system is composed of a hardware system, a software system and a functional layer, wherein the hardware system is composed of a patient end, a doctor end and a server end, the patient end is provided with an intelligent patient management engine, an intelligent patient abnormality analysis engine andan intelligent question answering robot, the doctor end is provided with a scientific research management system, a follow-up management system and a patient management system, the server end is composed of an EDC application server, an EDC follow-up server, an EDC data server and a WeChat server, the EDC application server is provided with an electronic medical record system, and the EDC data server is provided with a knowledge database management platform and a specialized disease knowledge database. The system is advantaged in that automatic information collection for pre-diabetes patientmanagement is achieved, real-time query and intelligent push of the prevention and control knowledge are achieved, doctors are assisted in management, research and clinical research, efficiency of managing patients is improved, and communication between the doctors and the patients is facilitated.
Owner:SHANGHAI CHANGHAI HOSPITAL
Compositions for the treatment of diabetes and pre-diabetes
ActiveUS8952068B2High levelImprove bioavailabilityBiocideMetabolism disorderAcute hyperglycaemiaHypertriglyceridemia
The invention relates to the compositions of formula I or its pharmaceutical acceptable polymorphs, solvates, enantiomers, stereoisomers and hydrates thereof. The pharmaceutical compositions comprises a salt of metformin and the methods for treating or preventing metabolic syndrome, prediabetes and diabetes may be formulated for oral, buccal, rectal, topical, transdermal, transmucosal, intravenous, parenteral administration, syrup, or injection. Such compositions may be used to treatment of diabetes mellitus, obesity, lipid disorders, hypertriglyceridemia, hyperglycemia, hyperinsulinemia and insulin resistance.
Owner:CELLIXBIO PTE LTD
Pharmaceutical composition, methods for treating and uses thereof
ActiveUS20160038524A1Few complianceFew convenienceBiocideMetabolism disorderPre diabetesKidney injury
The present invention relates to certain SGLT-2 inhibitors for treating and / or preventing metabolic disorders, such as type 1 or type 2 diabetes mellitus or pre-diabetes, in patients with renal impairment or chronic kidney disease (CKD).
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions for diabetes
InactiveUS20050113459A1Satisfactory controlHighly safe oral compositionBiocidePeptide/protein ingredientsAcute hyperglycaemiaIGT - Impaired glucose tolerance
The present invention provides a composition for improving glucose tolerance to patients having diabetic hyperglycemia and people having pre-diabetes, i.e., abnormal glucose tolerance. The composition helps control the blood glucose level, prevents diabetic complications, and can be administered over a long term due to its high safety. The composition containing a coenzyme Q, in particular, a reduced coenzyme Q10, as the main component improves the impaired glucose tolerance of diabetes patients, satisfactorily controls the blood glucose level, and decreases the blood glycosylated hemoglobin level.
Owner:KANEKA CORP
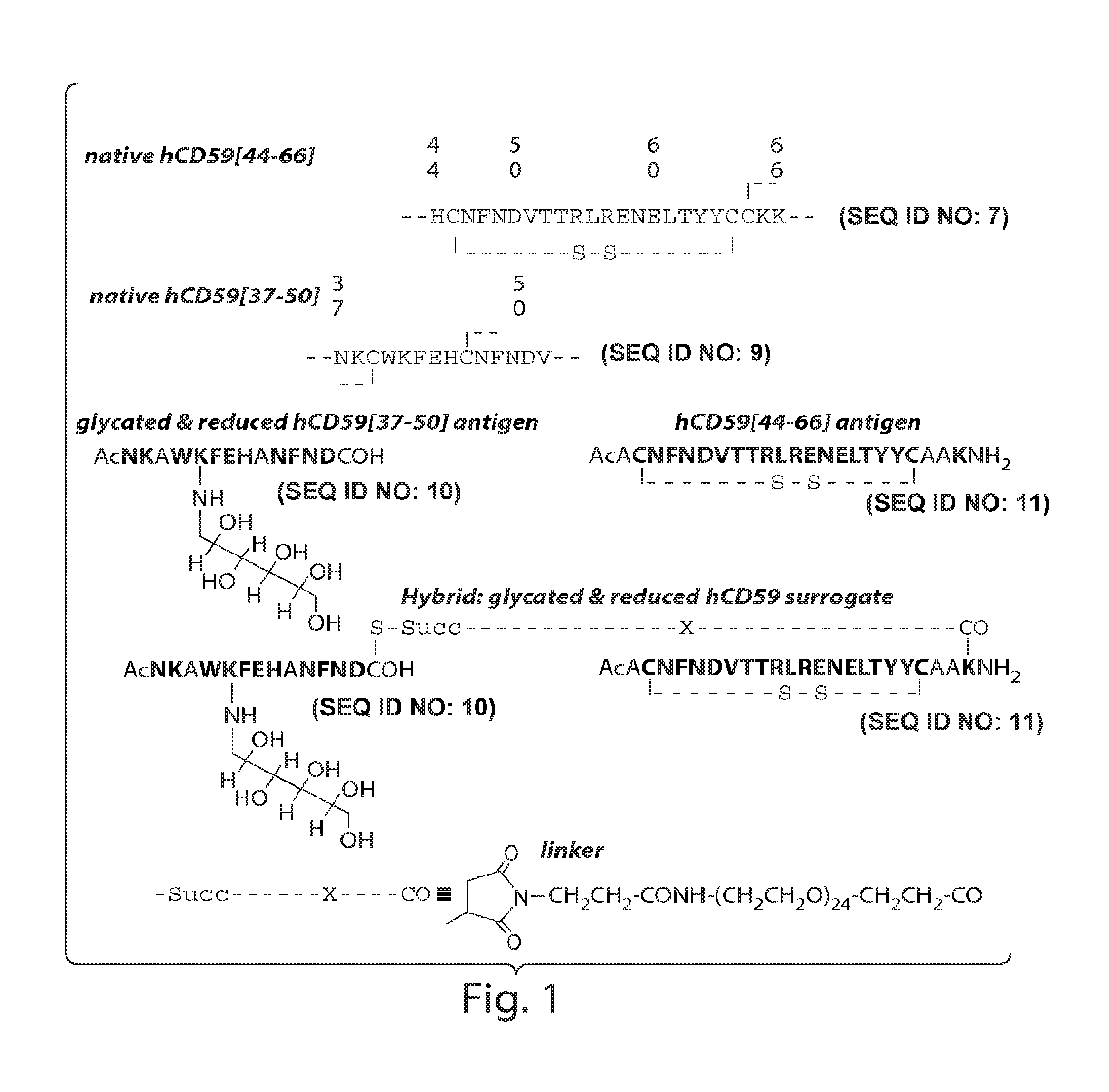
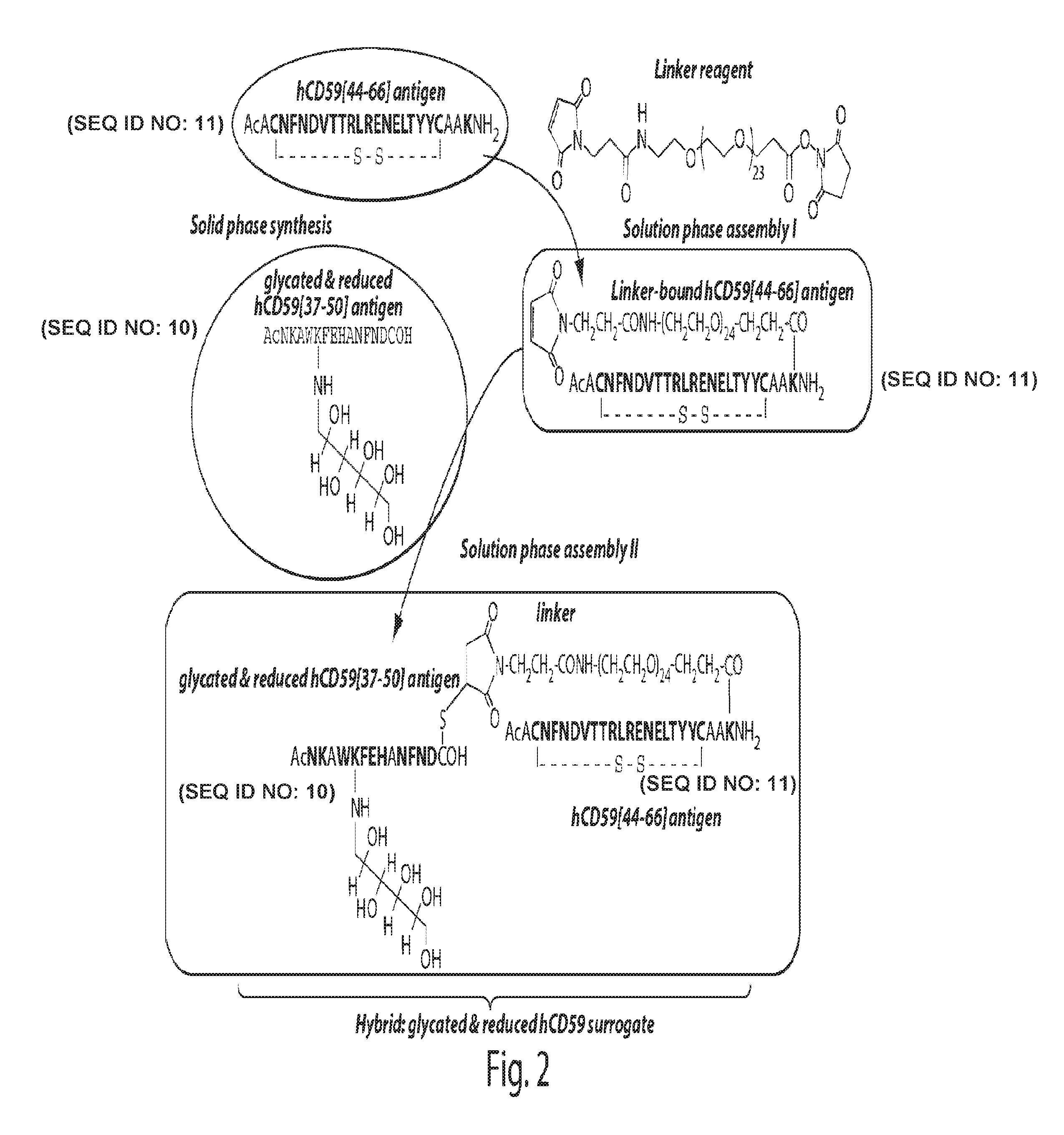
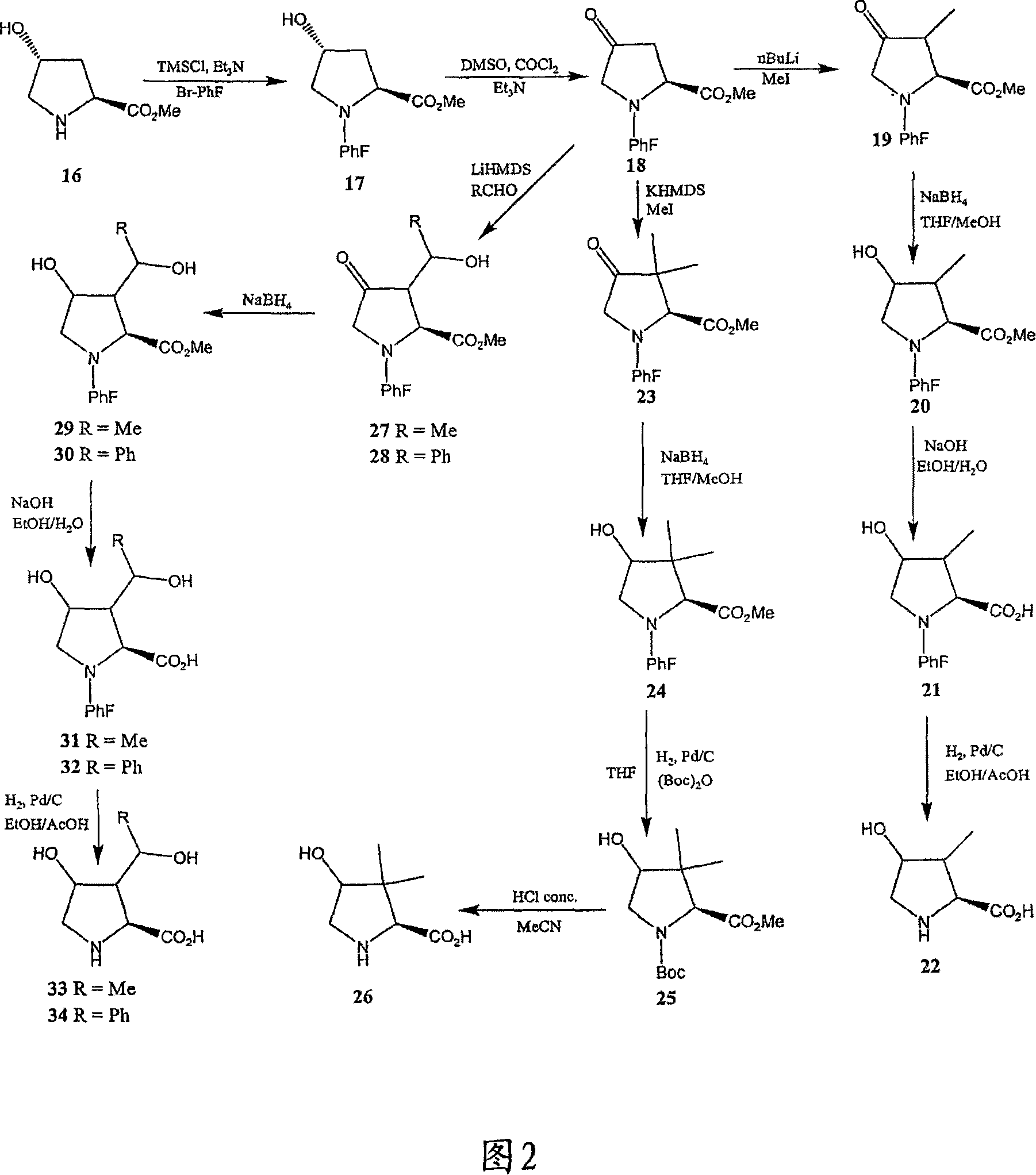
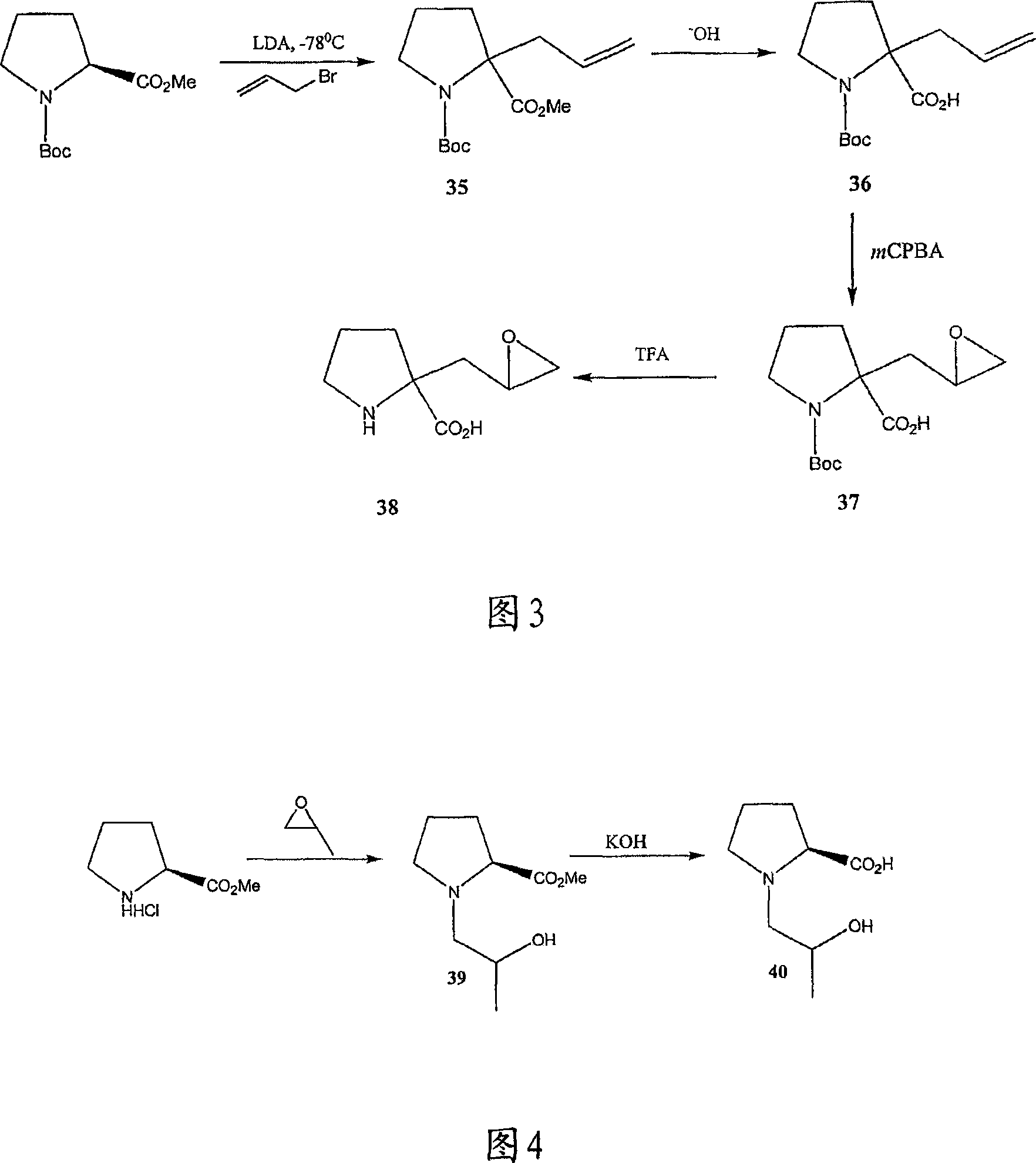
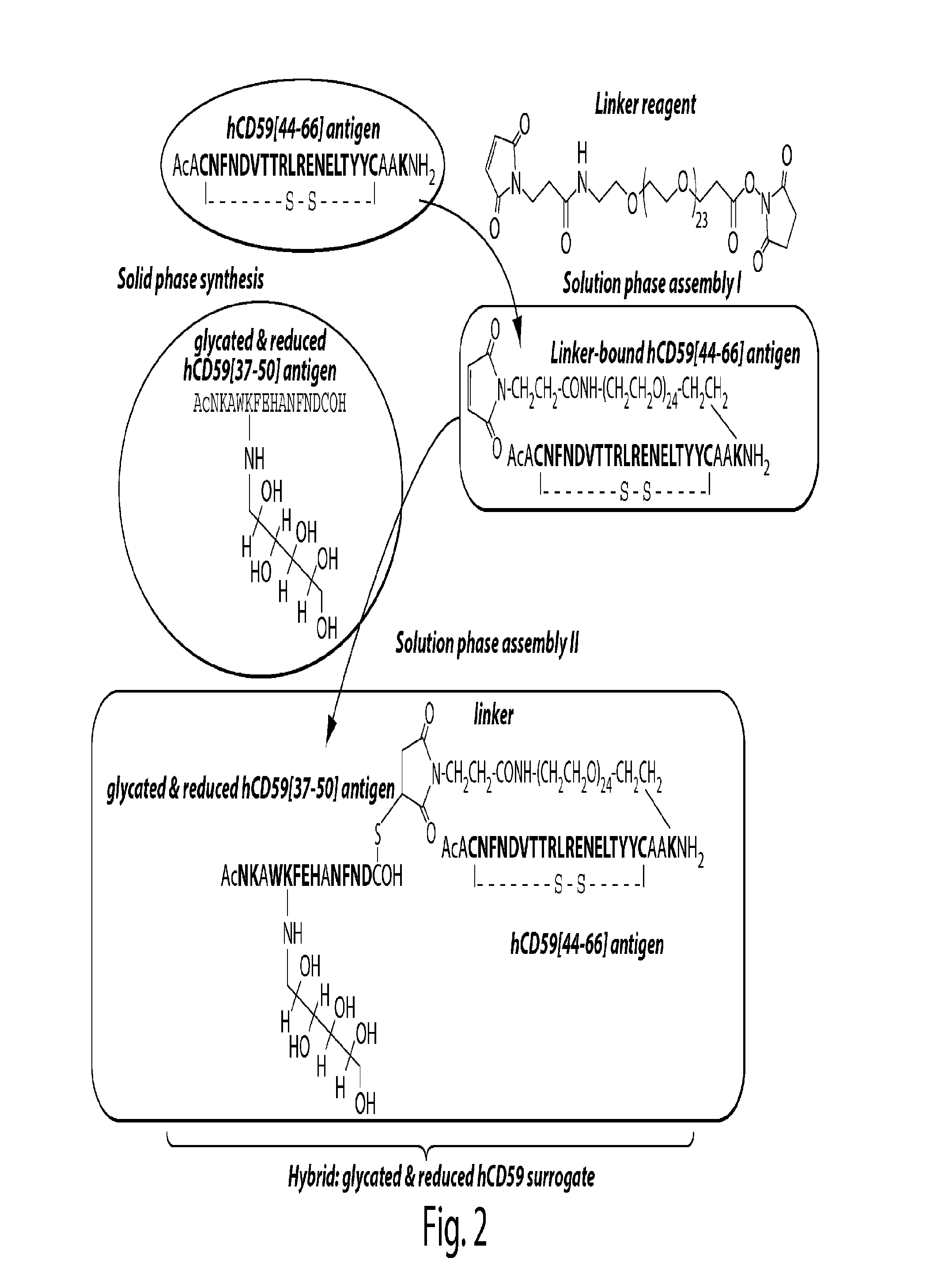
Surrogates of post-translationally modified proteins and uses thereof
ActiveUS9417248B2Cell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsCompound specificDisease
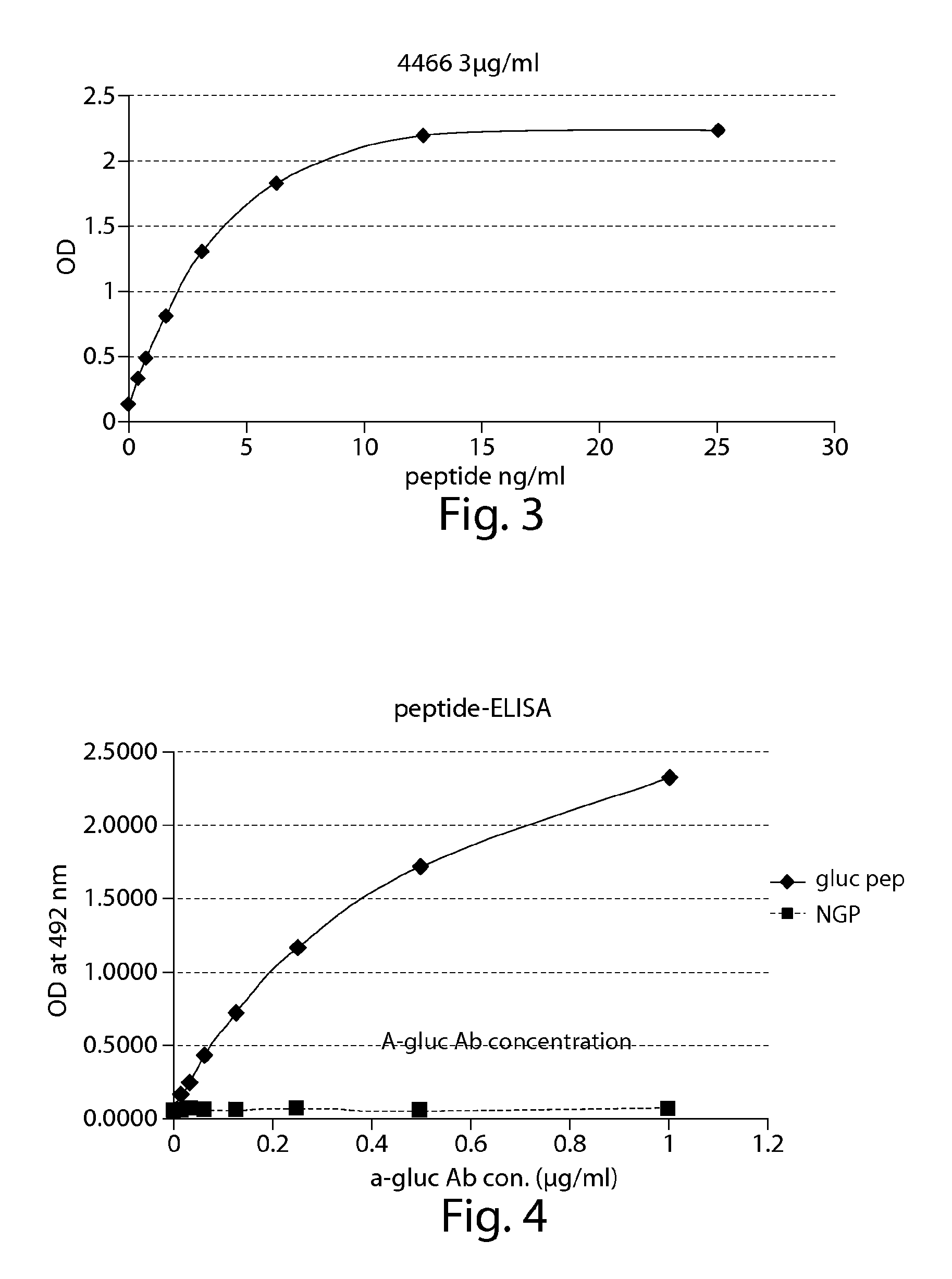
The present invention provides compounds that are surrogates of post-translationally modified proteins and uses thereof. Numerous diseases are associated with post-translationally modified proteins that are difficult to obtain in homogenous form and in quantities needed for immunization and use as convenient standards, calibrators, and / or reference compounds that facilitate the detection and analysis of endogenous post-translationally modified proteins. The surrogate compounds of the invention typically comprise antigenic epitopes (one of which carries a post-translational modification) that are tethered by a flexible and hydrophilic linker. The resulting compound behaves like a surrogate of the post-translationally modified protein because it preserves the character of the included antigens and allows recognition by specific antibodies targeting the individual antigens. The surrogate compounds may be prepared by covalently joining two or more polypeptide epitopes using one or more linkers, wherein at least one of the epitopes comprises a post-translational modification. In one aspect, the surrogate compounds of the invention comprise a C-terminal epitope and a glycated epitope of human CD59. The inventive methods allow quantification of the levels of glycated CD59 in the serum in human subjects, particularly those with diabetes or pre-diabetes. This technological platform of post-translationally modified protein surrogates can be applied to other diseases associated with post-translationally modified proteins (e.g., autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus). In another aspect, the invention provides antibodies that bind specifically to the compounds of the invention and methods for producing such antibodies.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Treatment of metabolic disorders in canine animals
ActiveUS20160361289A1Safe and effective treatmentReduce doseOrganic active ingredientsPowder deliveryInduced CataractPancreatic structure
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a canine animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, insulin dependent diabetes mellitus, insulin resistance diabetes, insulin resistance, obesity, hyperglycemia, hyperglycemia induced cataract formation, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, inflammation of the pancreas, metabolic disorder consequences, such as hypertension, renal dysfunction and / or muscoskeletal disorders, and / or Syndrome X (metabolic syndrome), wherein preferably the development of hyperglycemia induced cataract formation is prevented or remission is achieved and / or wherein preferably the development of metabolic disorder consequences, such as hypertension, renal dysfunction and / or muscoskeletal disorders, is prevented or progression is slowed or remission is achieved.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
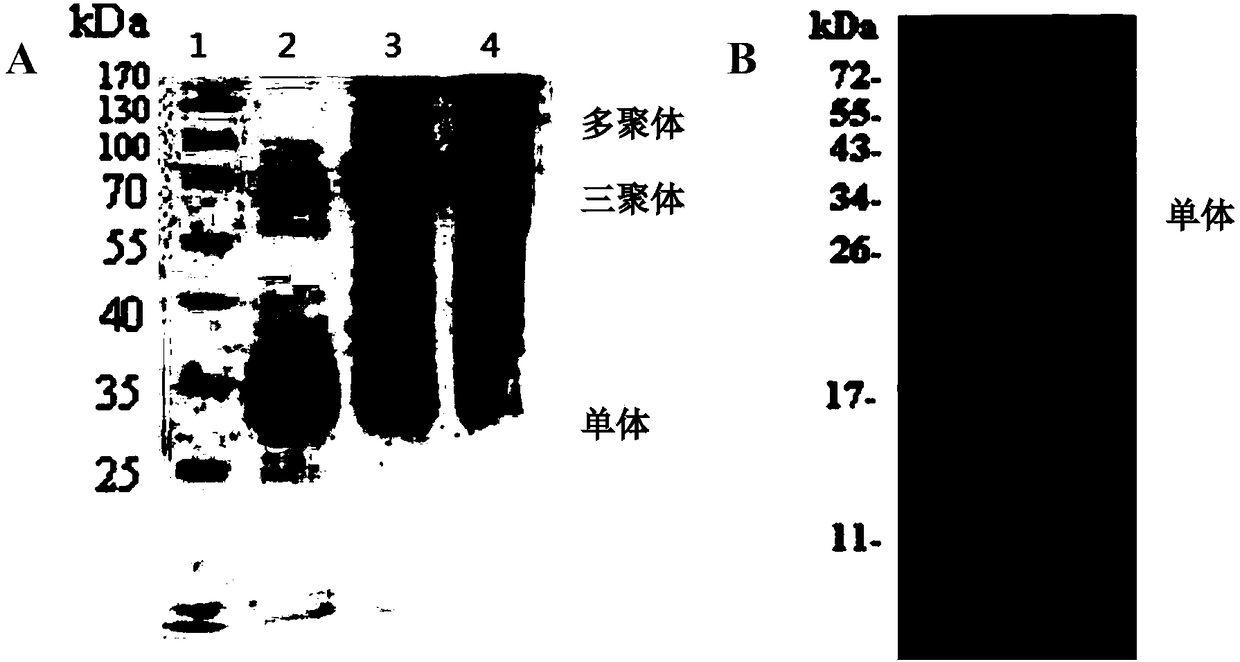
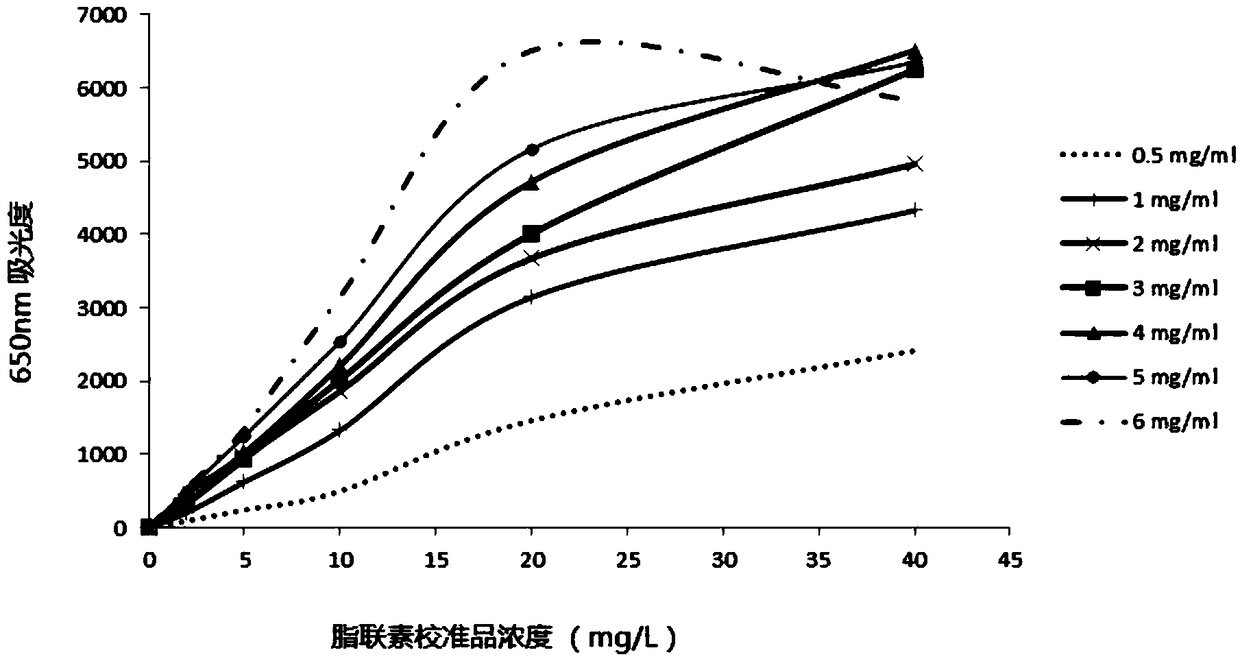
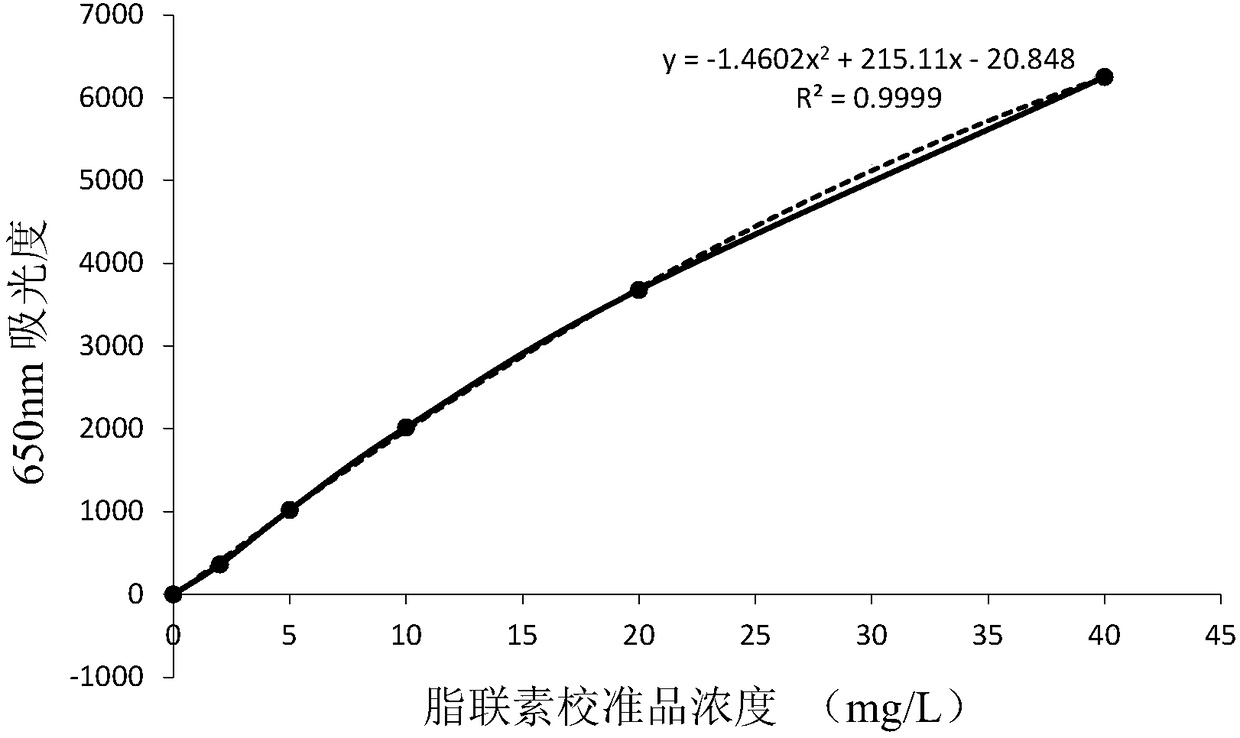
Recombinant adiponectin antigen, antibody and adiponectin nano latex-enhanced turbidimetric immunoassay kit
The invention discloses a recombinant adiponectin antigen. A preparation method of the recombinant adiponectin antigen includes the steps of a, culturing a high-expression recombinant human full-length adiponectin protein cell strain in a high-glucose DMEM culture medium under oxygen input quantity of 0.25-0.75L / minute, carbon dioxide quantity of 5-10%, bovine serum quantity of 5-10% and rotationspeed of 100-200rpm to allow the cell number of the cell strain to reach 1-3*10<7>; b, replacing the culture medium with a serum-free culture medium, adding 0.5% of vitamin C, and allowing the cell strain to keep the cell number of 1-3*10<7> and continuously express adiponectin for 4-10 days. The invention further discloses an adiponectin antibody, adiponectin antibody nano latex particles and a kit containing the antigen and the antibody nano latex particles. The kit using the nano latex-enhanced turbidimetric immunoassay to detect adiponectin is high in accuracy, real and reliable in data and good in predicting effect on type 2 diabetes and pre-diabetes.
Owner:广东英诺生物科技有限公司
AMPK activating compound and its use
InactiveCN104447746ALower plasma glucoseLower triglyceridesOrganic chemistryMetabolism disorderDiseasePre diabetes
The invention relates to an AMPK activating compound and its use in the preparation of drugs for preventing or treating diseases or physiological states which can be improved by an AMPK activator and comprise pre-diabetes, insulin resistance, type II diabetes, X-syndromes, metabolism syndromes and obesity. The compound reduces the blood plasma glucose amount by above 30wt%, reduces the triglyceride amount by above 35wt% and reduces the body weight by above 15%.
Owner:ENERGENESIS BIOMEDICAL
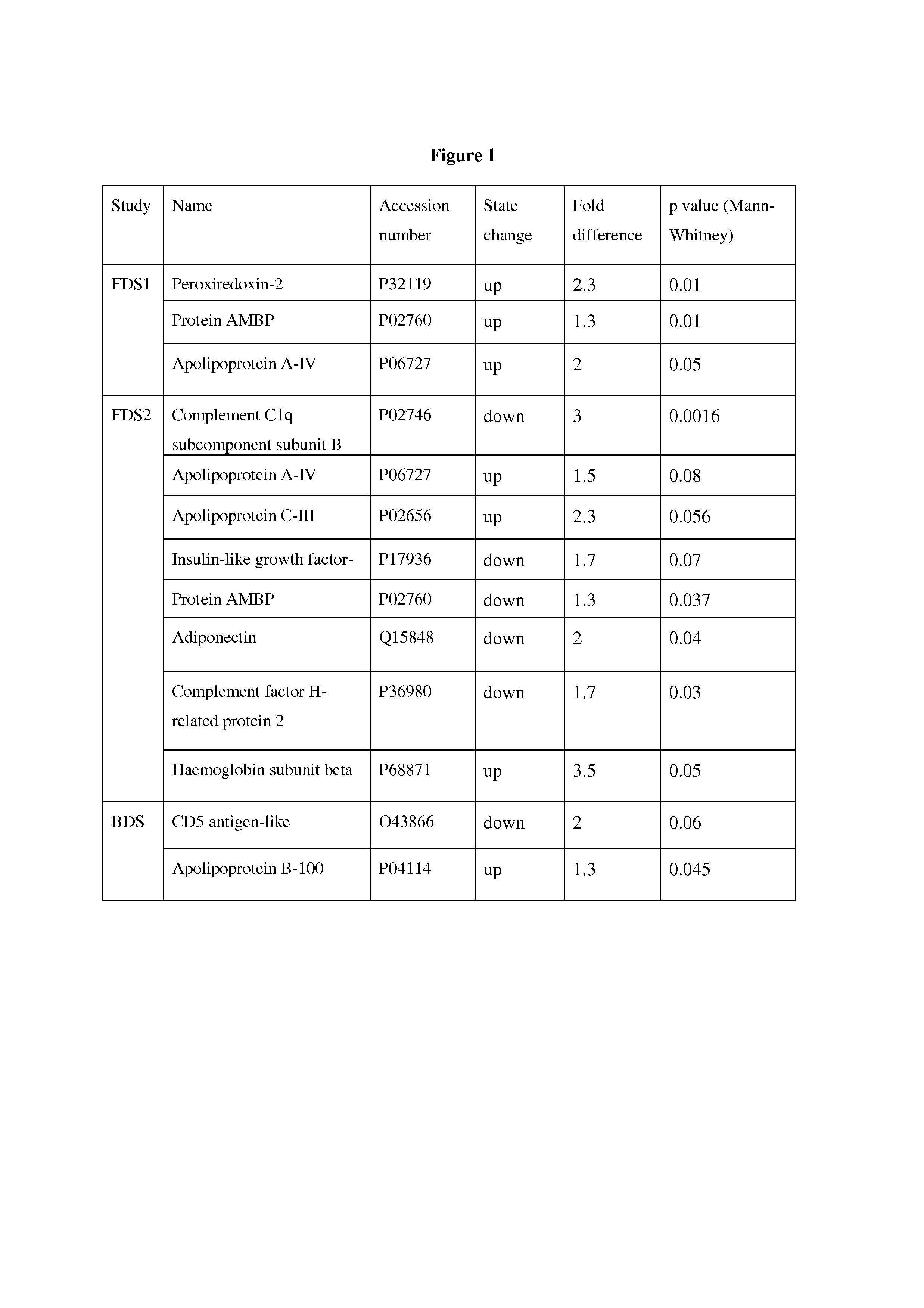
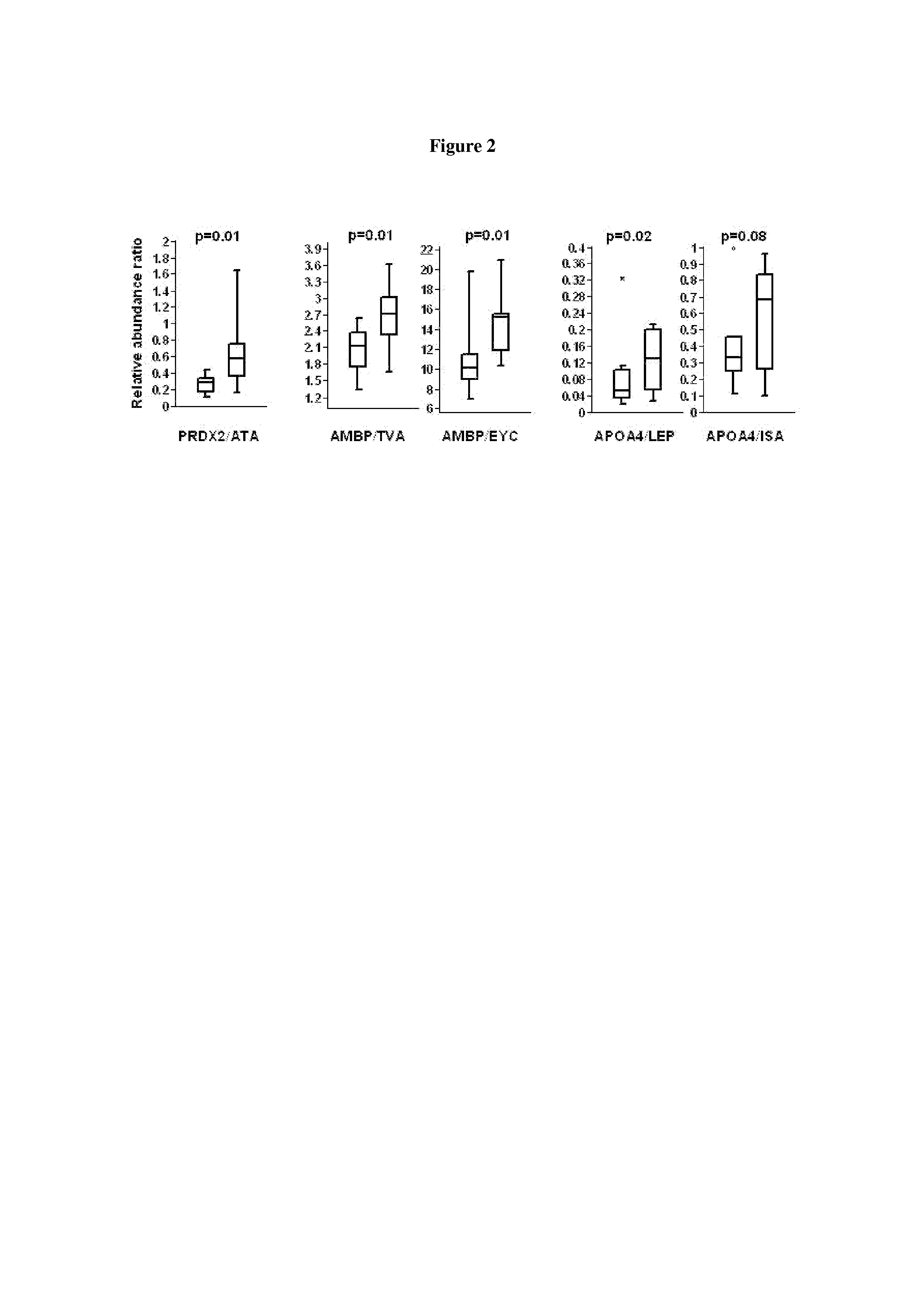
Biomarkers associated with pre-diabetes, diabetes and diabetes related conditions
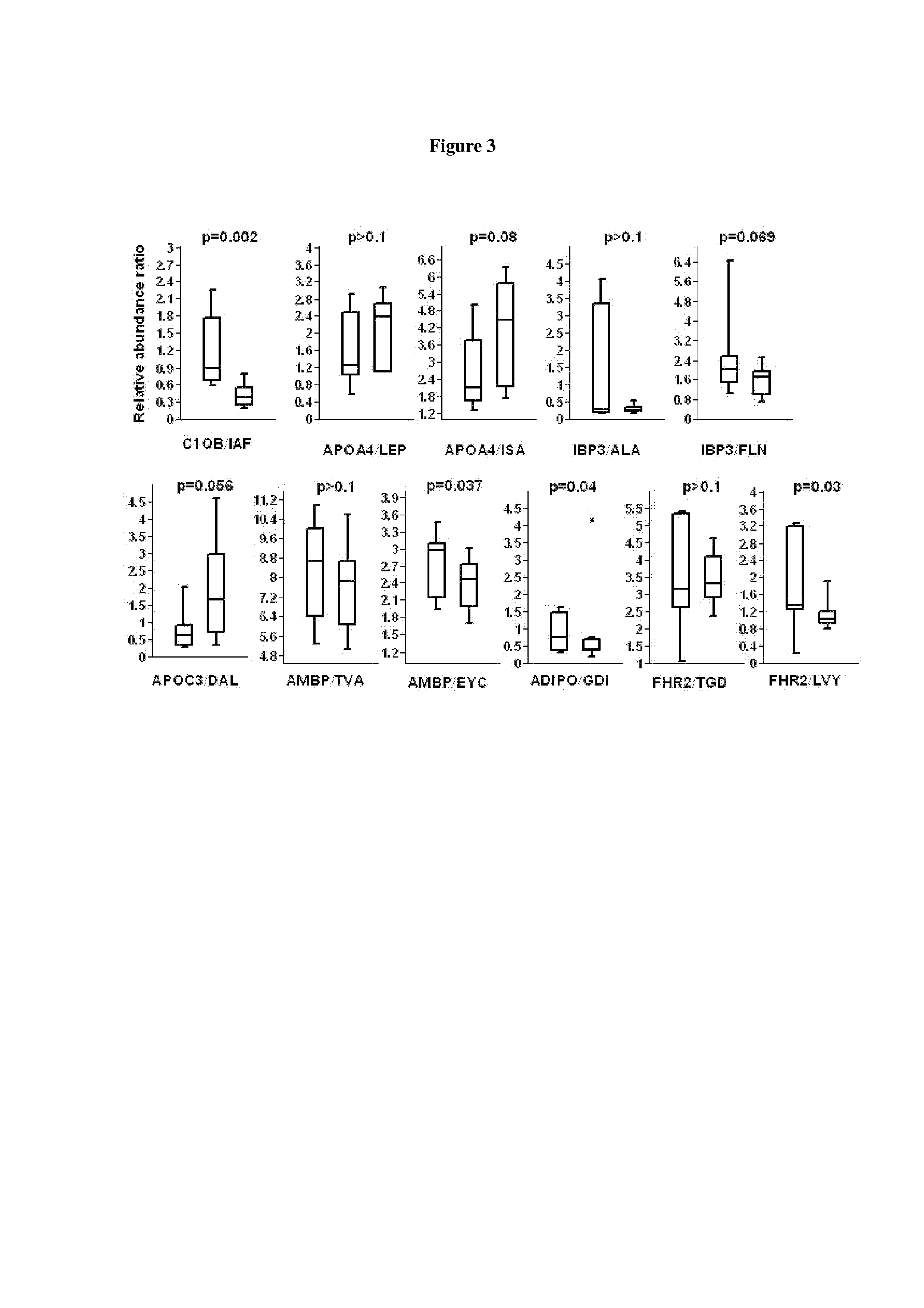
InactiveUS20130177544A1Reduce development riskReduce riskBioreactor/fermenter combinationsBiological substance pretreatmentsComplement C1qDisease
Biomarkers for pre-Diabetes, Diabetes and / or a Diabetes related conditions, and methods of their use, including the biomarkers in Tables 1 and 2 such as peroxiredoxin-2, complement C1q subcomponent subunit B, sulfhydryl oxidase 1 and apolipoprotein A-IV.
Owner:PROTEOMICS INT +1
Method for treatment of diabetes by electrostimulatioin
A method for treating a patient suffering diabetes or pre-diabetes is disclosed. In brief, this treatment involves delivering a micro-current to the patient, in accordance with a defined therapeutic regime. More specifically, this invention relates to the treatment of diabetes, by means of electrostimulation, so as to correct / abate the biological processes which are believed to be destructive of the cells involved in the production of insulin. In practice a body conformable, electrode wrap is secured to an individual, and an alternating, direct current applied to the electrode wrap, within the range of from 20 milli-amperes to 1 atto-ampere (1×10−18 amps). The micro current is preferably an alternating direct current having a frequency in the range of from 0.00065 Hz to 0.00085 Hz. This therapeutic regime is effective in the stabilization of blood sugar levels in insulin dependent diabetics.
Owner:WENDELL KEITH F
Edible blend oil for prevention and treatment of pre-diabetes and preparation method thereof
InactiveCN109169961AImprove dietary structureNutritional balanceEdible oils/fatsAdditive ingredientAcute cardiovascular disease
The invention discloses an edible blend oil for prevention and treatment of pre-diabetes, and belongs to the field of blend oil manufacturing technology. The edible blend oil for prevention and treatment of pre-diabetes includes the following ingredients: soybean oil, olive oil, seal oil, omega-3 fatty acid, omega-6 fatty acid, essential amino acid, fat-soluble vitamin, water-soluble vitamin, trace element, fructose, glucose, carotene, chlorophyll, etc. The edible blend oil for prevention and treatment of pre-diabetes provided by the invention can replace traditional peanut oil for eating. Theedible blend oil for prevention and treatment of pre-diabetes contains a variety of beneficial ingredients, has more balanced nutrition, and contains a large number of linoleic acid, linolenic acid and unsaturated acid, alpha-linolenic acid, olive polyphenols, etc. The edible blend oil for prevention and treatment of pre-diabetes is low in cholesterol content and good for patients with hyperlipemia, hypertension, hyperglycemia, obesity and cardiovascular and cerebrovascular diseases after long-term consumption. The edible blend oil for prevention and treatment of pre-diabetes has good antioxidant and free radical scavenging abilities, and the edible blend oil is beneficial to the prevention of diabetic cardiovascular diseases and good in blood sugar lowering effect.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
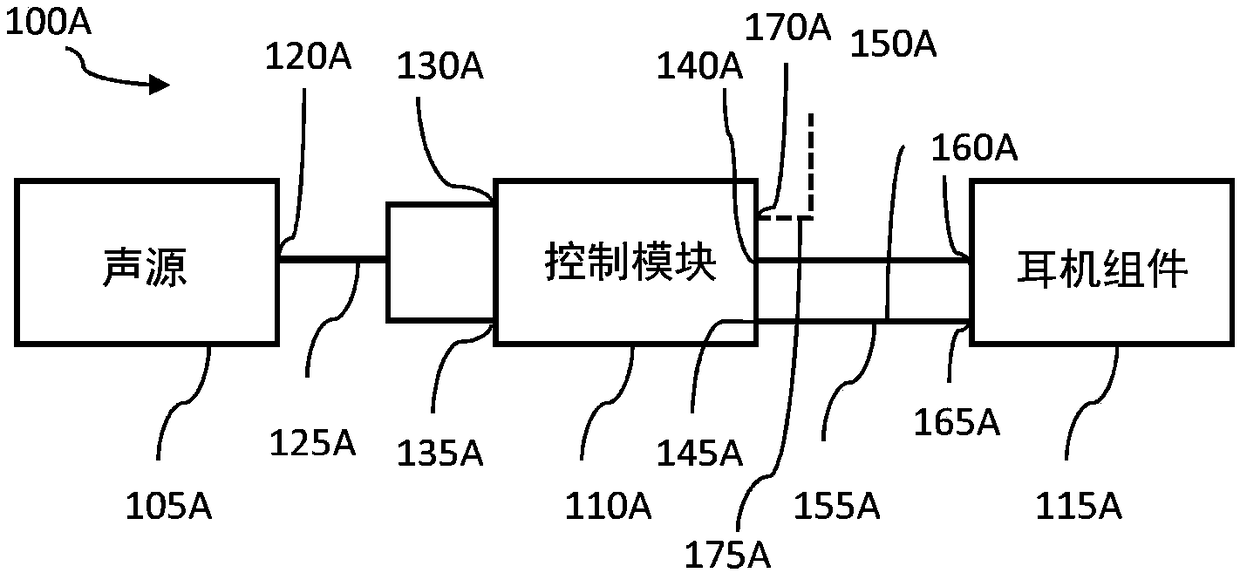
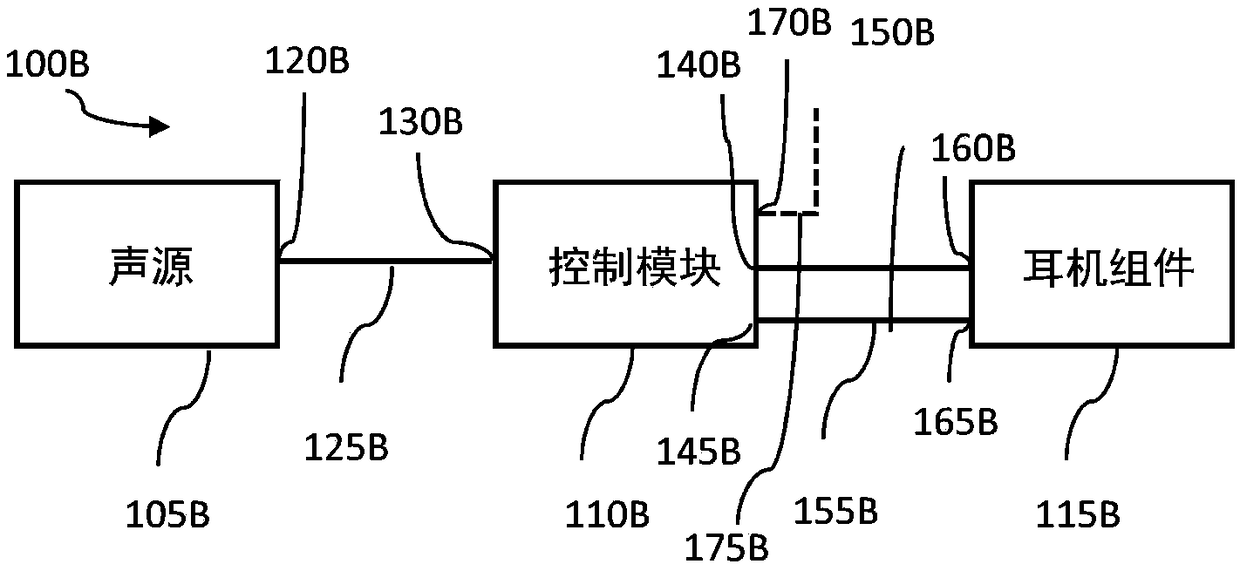
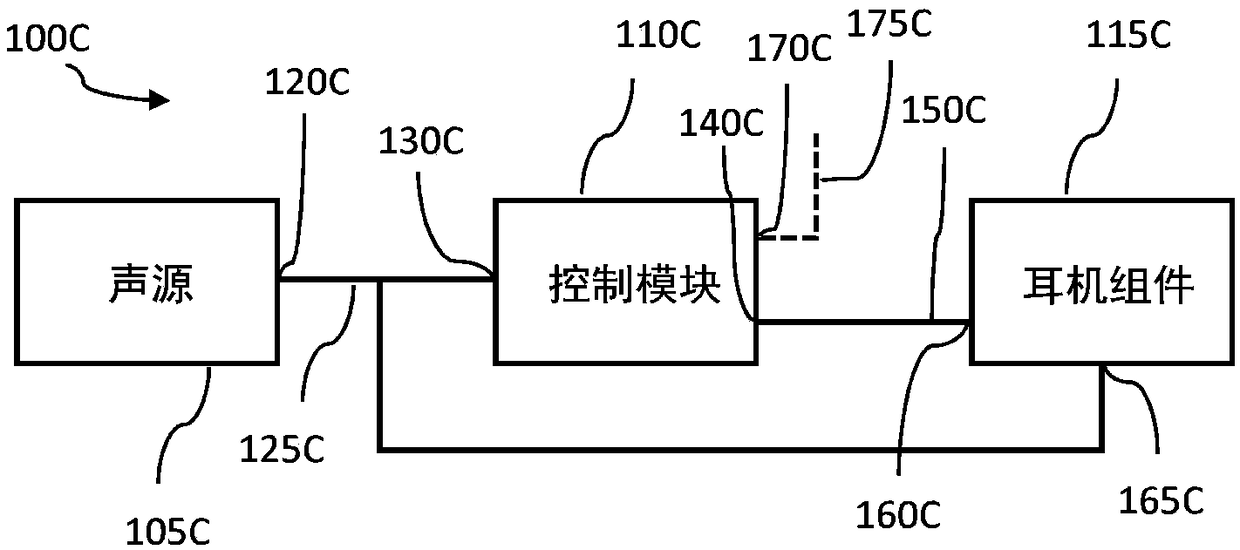
System and method for ear-arranged transcutaneous vagus nerve stimulation
InactiveCN109069297AConvenient treatmentImprove convenienceElectrotherapyMedical devicesSound sourcesEngineering
A transcutaneous nerve stimulation system includes a multifunctional earphone assembly that receives a sound signal from a sound source or other sources (such as a signal generator). An amplifier receiving the sound signal from the sound source or independent signal generator amplifies the sound signal to generate an amplified electrical signal. A stimulator, which includes multiple conductive electrode contacts, is coupled to the amplifier to receive the amplified electrical signal. The electrode contacts may protrude from an earbud / headphone or extend from an adjustable probe arm. The amplified electrical signal is used to apply electrical stimulation while the earphone assembly emits audible sounds according to the sound signal. The combined functionality enhances compliance with treatment regimens involving electrical stimulation of vagus nerves and other regions of a subject. Potential indications include, without limitation, major depressive disorder, epilepsy, chronic pain, pre-diabetes, insomnia, cardiovascular disorders, tinnitus, autism, daily stress, and anxiety.
Owner:THE GENERAL HOSPITAL CORP
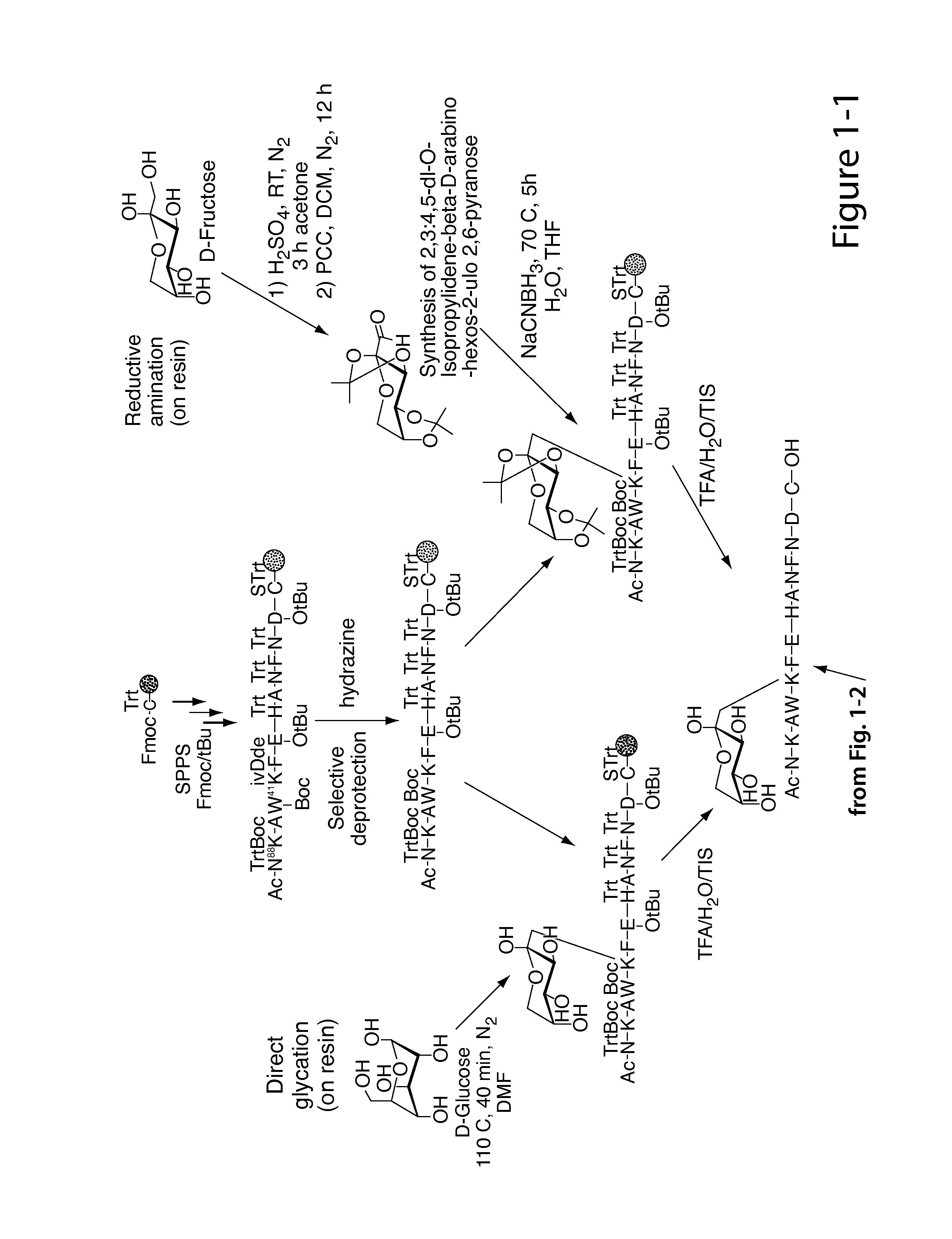
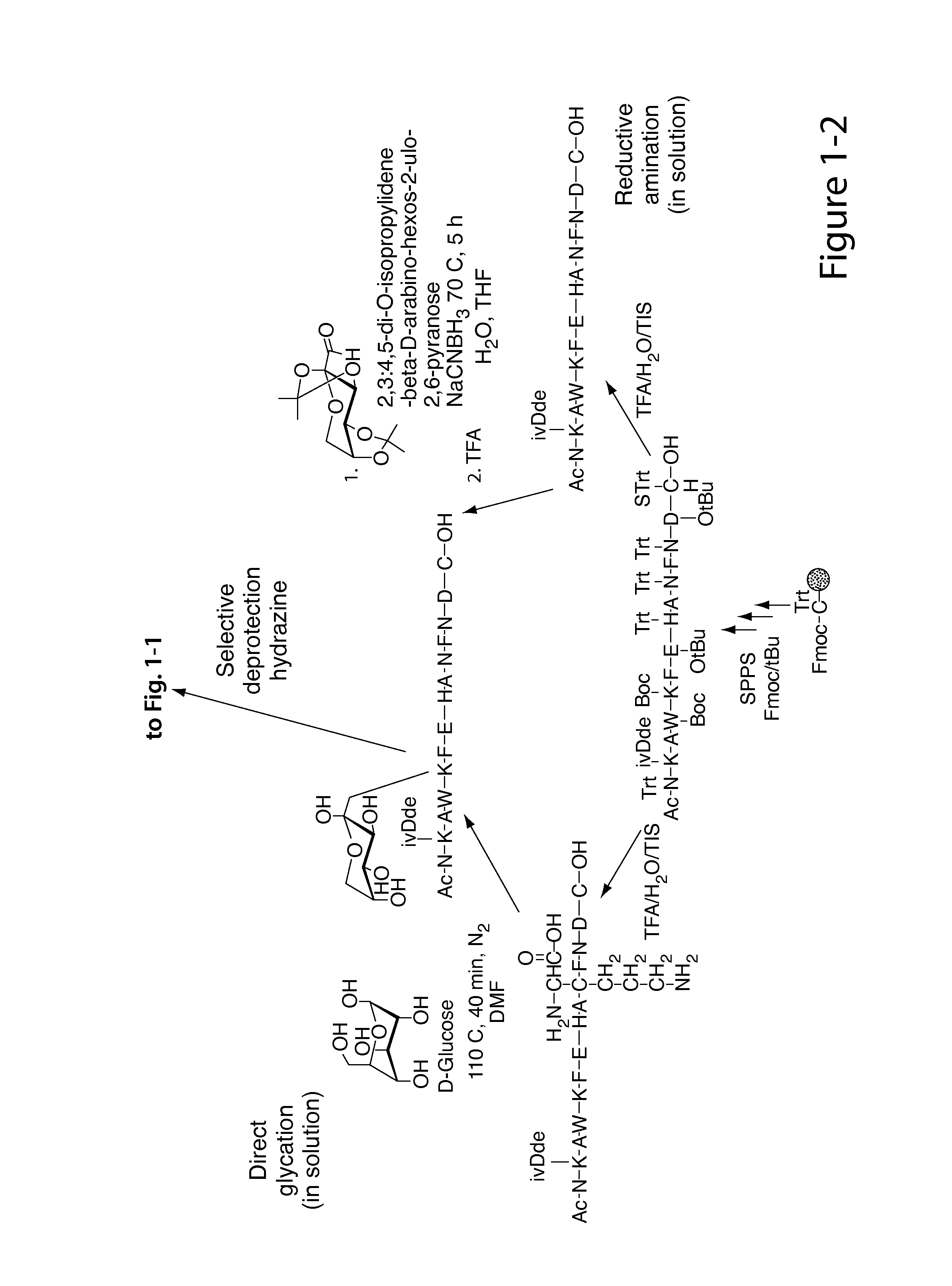
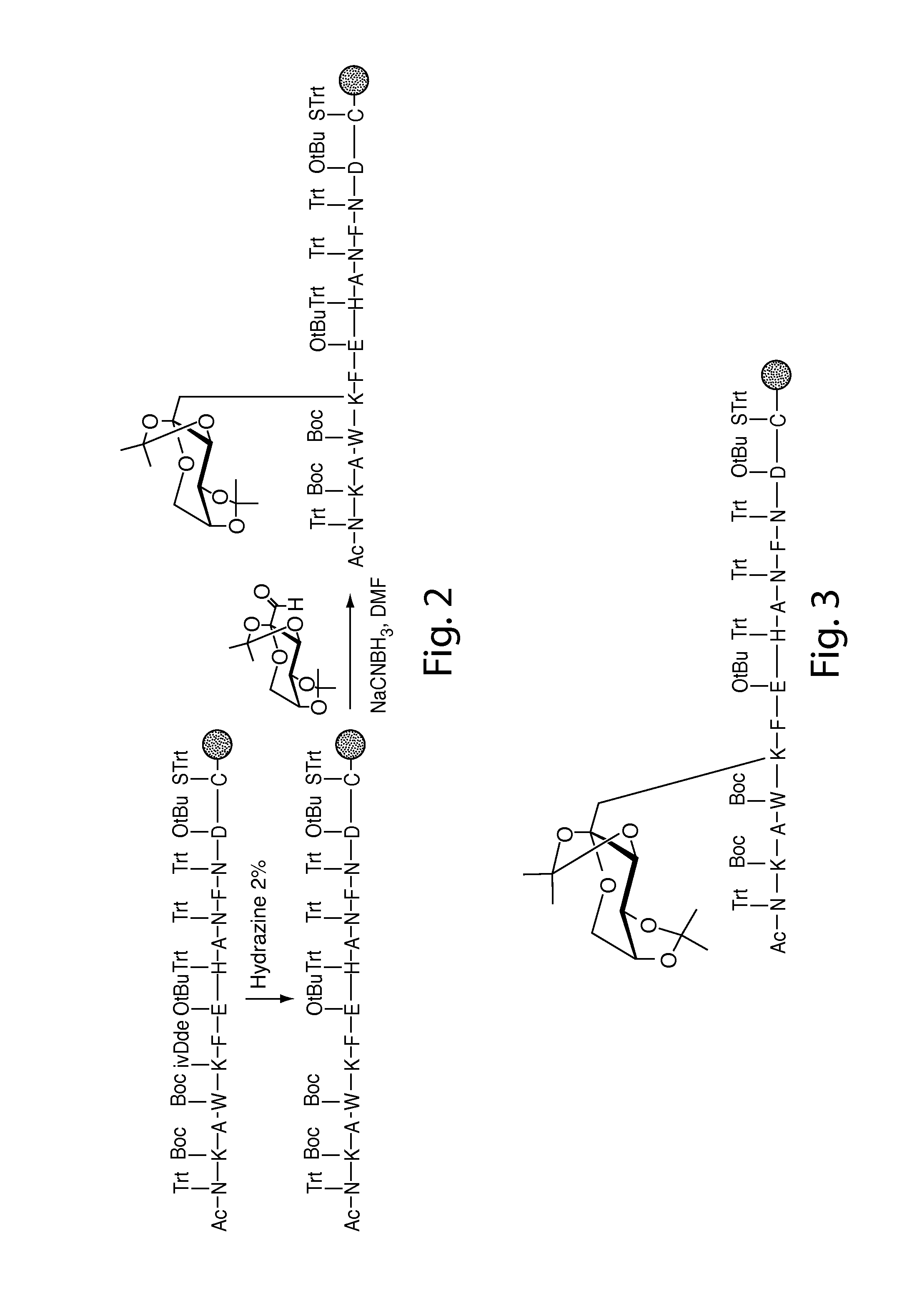
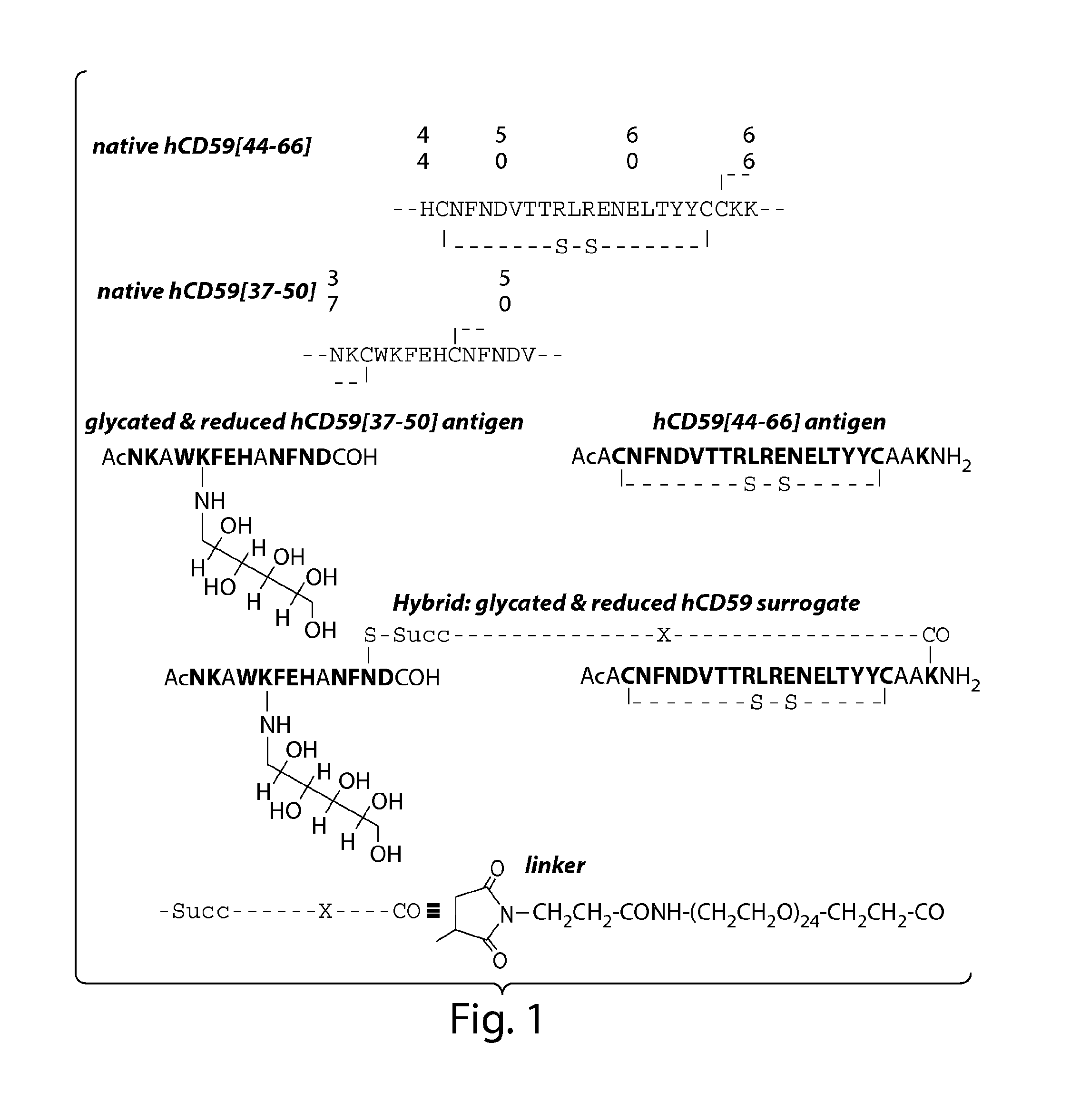
Glycated CD59 peptides, their preparation, and uses thereof
ActiveUS9068006B2Activation of and excessive and acceleratedExcessive and accelerated depositionPeptide/protein ingredientsGenetic material ingredientsAntibody fragmentsPre diabetes
The present invention provides glycated Amadori products of the CD59 peptide and fragments thereof to be used as tools and among methods for the diagnosis and prognosis of pre-diabetes and diabetes. Certain aspects of the invention include glycated Amadori products of CD59 and fragments thereof to be used for the generation of antibodies and antibody fragments. Still other aspects of the invention include methodologies for the preparation of glycated Amadori products of CD59, fragments thereof, the inventive antibodies, and antibody fragments.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Pharmaceutical composition for transmucosal delivery and methods for treating diabetes in a subject in need thereof
InactiveCN106794146APeptide/protein ingredientsPharmaceutical non-active ingredientsPre diabetesInsulin humulin
The present disclosure relates to pharmaceutical compositions of solid dosage form for intraoral administration that provides effective delivery of insulin and insulin analogs via the transmucosal route. Also provided are methods for treating pre-diabetes, diabetes and metabolic syndrome in a subject in need thereof.
Owner:EASTGATE PHARMA
Analogs of 4-hydroxyisoleucine and uses thereof
The invention relates to analogs of 4-hydroxyisoleucine, and to lactones, pharmaceutically acceptable salts, and prodrugs thereof, to processes for their preparation, and to pharmaceutical compositions comprising the same. The analogs of the invention stimulate both glucose uptake and insulin secretion, and might thus be useful for the prevention and treatment of disorders of carbohydrate or lipid metabolism, including diabetes mellitus (type 1 and type 2 diabetes), pre-diabetes, and Metabolic Syndrome.
Owner:INNODIA INC +2
Surrogates of post-translationally modified proteins and uses thereof
ActiveUS20140024056A1Cell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsCompound specificDisease
The present invention provides compounds that are surrogates of post-translationally modified proteins and uses thereof. Numerous diseases are associated with post-translationally modified proteins that are difficult to obtain in homogenous form and in quantities needed for immunization and use as convenient standards, calibrators, and / or reference compounds that facilitate the detection and analysis of endogenous post-translationally modified proteins. The surrogate compounds of the invention typically comprise antigenic epitopes (one of which carries a post-translational modification) that are tethered by a flexible and hydrophilic linker. The resulting compound behaves like a surrogate of the post-translationally modified protein because it preserves the character of the included antigens and allows recognition by specific antibodies targeting the individual antigens. The surrogate compounds may be prepared by covalently joining two or more polypeptide epitopes using one or more linkers, wherein at least one of the epitopes comprises a post-translational modification. In one aspect, the surrogate compounds of the invention comprise a C-terminal epitope and a glycated epitope of human CD59. The inventive methods allow quantification of the levels of glycated CD59 in the serum in human subjects, particularly those with diabetes or pre-diabetes. This technological platform of post-translationally modified protein surrogates can be applied to other diseases associated with post-translationally modified proteins (e.g., autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus). In another aspect, the invention provides antibodies that bind specifically to the compounds of the invention and methods for producing such antibodies.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com