AMPK activating compound and its use
A compound, mammalian technology, applied in the field of compounds to solve problems such as lactic acidosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
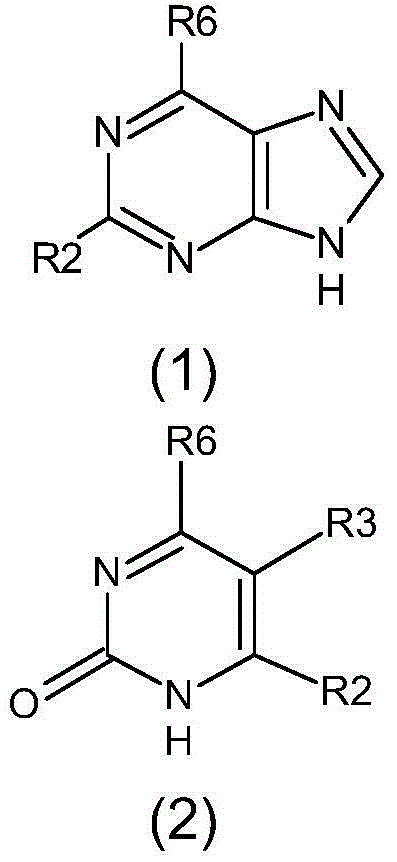
[0046] 2-Amino-6-(3-chlorobenzylamino)purine
[0047] After dissolving 4 mmol of 2-amino-6-chloropurine in 20 ml of butanol, 5 mmol of 3-chlorobenzylamine and 6 mmol of triethylamine were added. The mixture was reacted at 90°C for 4 hours. After cooling, the product was obtained by filtration, washed with water and butanol and crystallized from dimethylformamide or ethanol. HPLC: more than 98% purity. Productive rate 95%, the results are shown in Table 1.
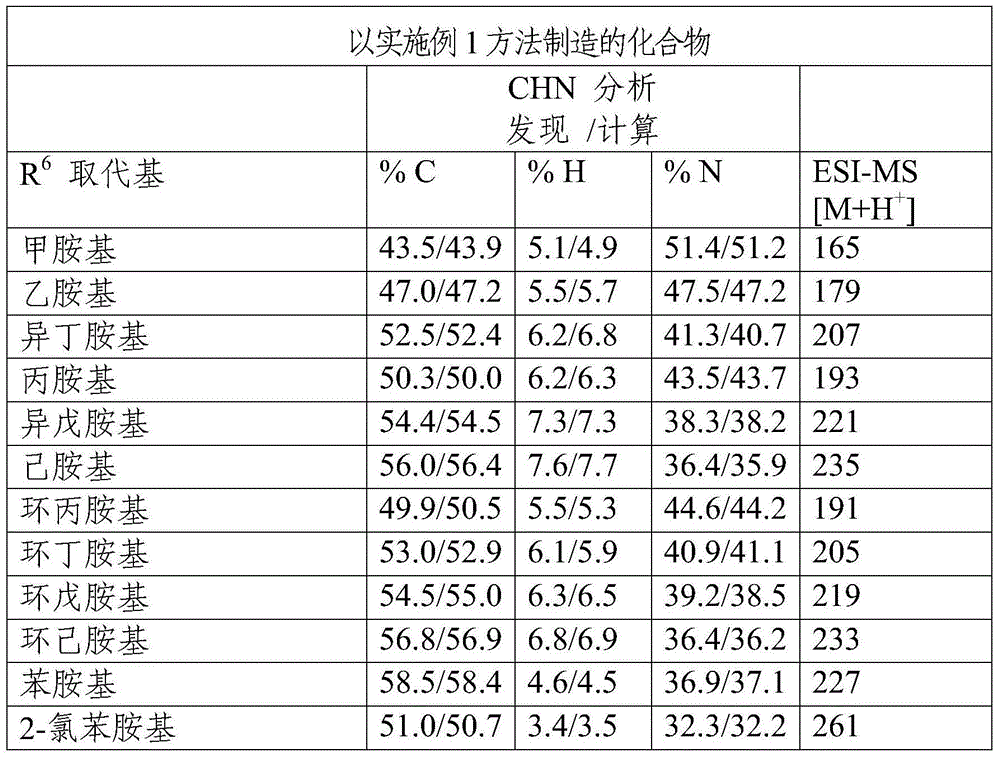
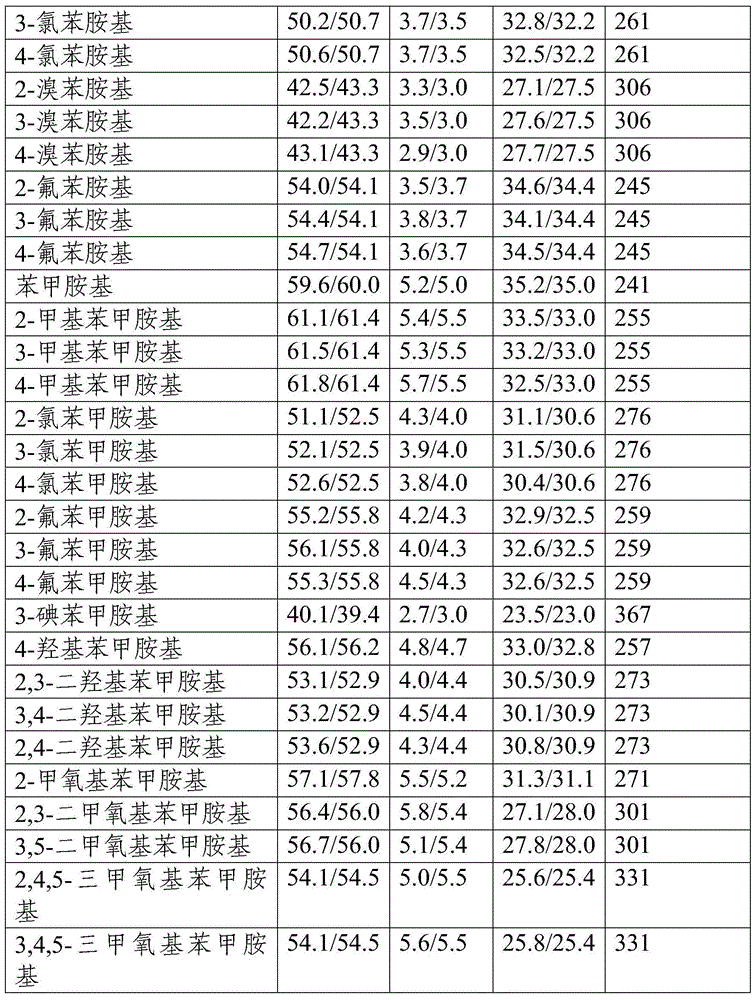
[0048] Table 1 is produced by the compound of embodiment 1 method
[0049]
[0050]
Embodiment 2
[0052] 6-(3-Chlorobenzylamino)purine
[0053]After dissolving 4 mmol of 6-chloropurine in 20 ml of butanol, 5 mmol of 3-chlorobenzylamine and 6 mmol of triethylamine were added. The mixture was reacted at 90°C for 4 hours. After cooling, the product was obtained by filtration, washed with water and butanol and crystallized from dimethylformamide or ethanol. HPLC: more than 97% purity. Productive rate 94%, the results are shown in Table 2.
[0054] Table 2 is manufactured with the compound of embodiment 2 method
[0055]
[0056]
Embodiment 3
[0058] 2-Hydroxy-6-chloropurine
[0059] After dissolving 4 mmol of 2-amino-6-chloropurine in 35 ml of 50 wt% sulfuric acid, 5 mmol of sodium nitrate was added. The mixture was reacted at -10°C for 2 hours and then at 50°C for 1 hour. After cooling, the product was obtained by filtration, washed with water and butanol and crystallized from dimethylformamide or ethanol. HPLC: more than 98% purity. Yield 86%. MS(ESI)m / e170.88(M+H + );1H NMR(DMSO-d6):8.01(s,1H,=CH-N),13.26(s,2H,OH and NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com