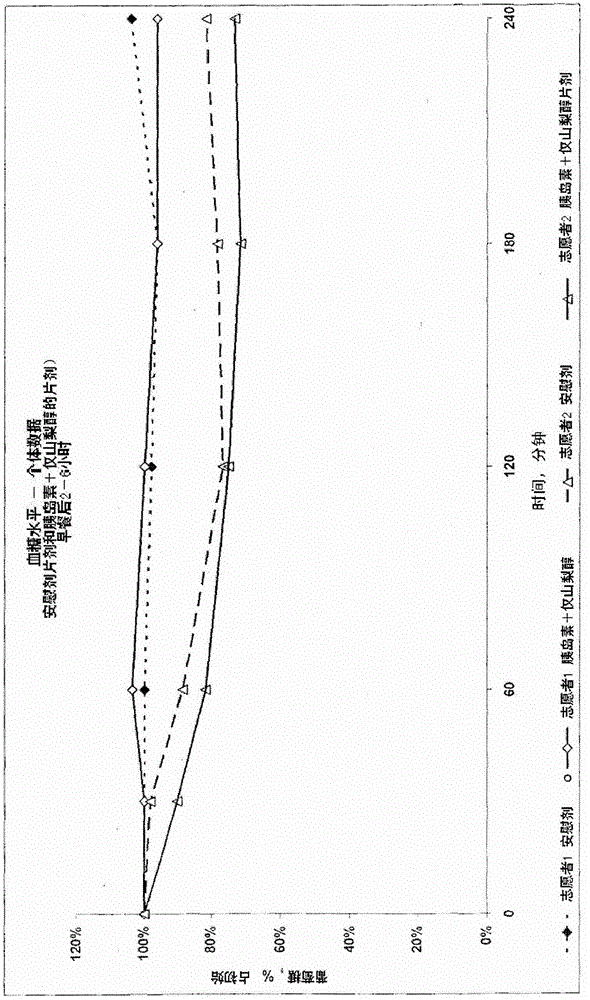
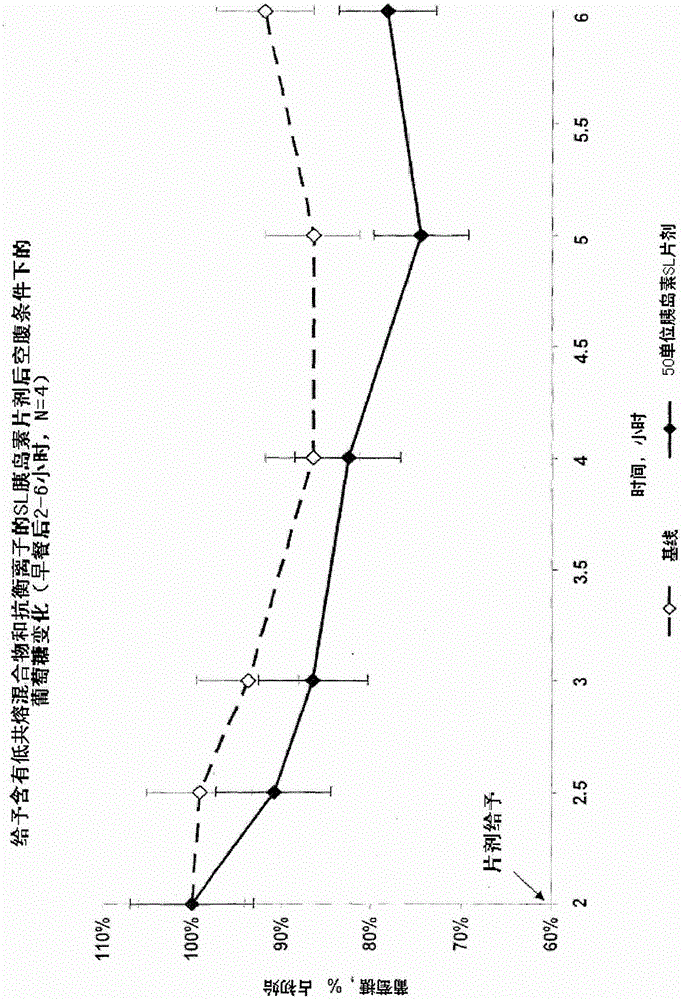
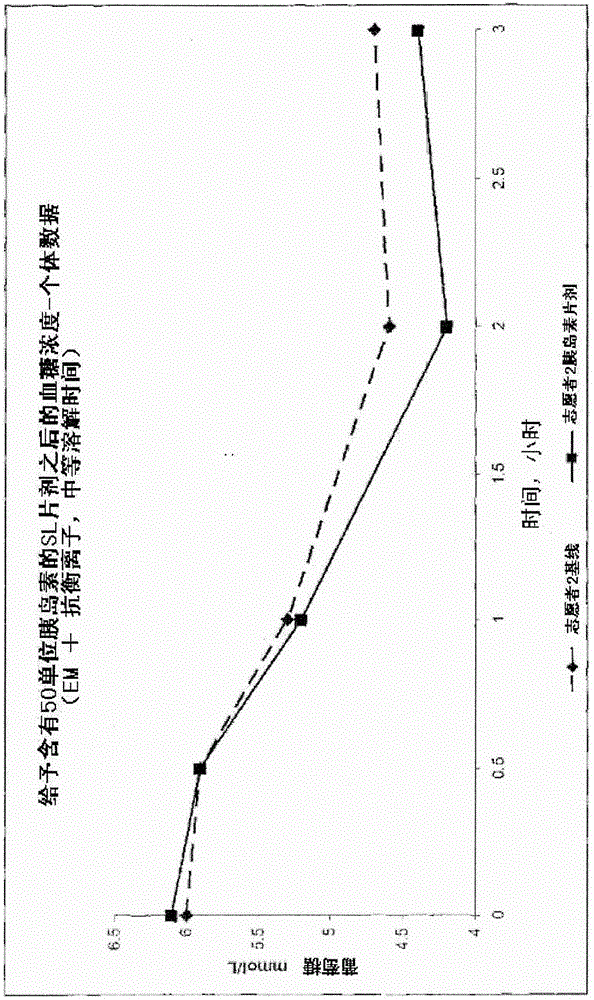
Pharmaceutical composition for transmucosal delivery and methods for treating diabetes in a subject in need thereof
A composition, transmucosal technology for use in the field of pharmaceutical compositions for transmucosal delivery and for the treatment of diabetes in a subject in need
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Embodiment 1: comparative agent
[0183] Sorbitol 20.0g ( P, Cerestar, US or P 150DC, Roquette, France) was carefully mixed with 196 mg of crystalline insulin (insulin USP, water content 9.5%, 27.7 units / mg of dry material). 500 mg of fine powdered PEG-3350NF was added and mixed well, then circular concave tablets (207 mg weight, 8 mm diameter) were compressed using a single punch tablet press. The hardness of the formed tablet was 10-14 kP and the tablet melted in the mouth within 4-7 minutes.
[0184] Placebo tablets were prepared in a similar manner but without insulin. The tablets formed (205 mg weight, 8 mm diameter) had a hardness of 10-13 kP. Tablets dissolve in the mouth within 4-7 minutes. Table 2 shows the composition (in mg) per tablet of the compressed tablets containing insulin but without eutectic mixture.
[0185] Table 2. Insulin Compressed Tablets Without Eutectic Mixture (Composition Per Tablet, mg)
[0186]
Embodiment 2
[0187] Example 2: Sublingual Insulin Tablets Containing Phospholipids
[0188] Using xylitol ( DC, Cerestar), microcrystalline cellulose ( 102), colloidal silicon dioxide and purified soybean lecithin to prepare tablets. After combining with crystalline insulin and sucralose, the blend was mixed carefully, sieved, then blended with PEG-3350 and compressed into round (8 mm) concave tablets (hardness 8-10 kP). The formed tablet dissolves in the mouth within 8-10 minutes.
Embodiment 3
[0189] Example 3: Sublingual Insulin Tablet Containing Soy Lecithin and Nonionic Surfactant
[0190] Tablets prepared as described in Example 2, but containing a combination of lecithin and selected nonionic surfactants.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com