Analogs of 4-hydroxyisoleucine and uses thereof
A technology of group and alkyl group, applied in the field of 4-hydroxyisoleucine analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0238] Acceptable methods of preparing pharmaceutical compositions in appropriate pharmaceutical forms are known to those skilled in the art. For example, pharmaceutical formulations can be prepared by conventional techniques of medicinal chemists involving steps such as mixing, granulation and compression (essential for tablets) to produce the desired product for each route of administration. ), or mix, fill and properly dissolve ingredients.
[0239] Toxicity and therapeutic efficacy of analogs according to the invention can be assessed by standard pharmaceutical procedures in cell culture or experimental animals. Therapeutic efficacy of analogs according to the invention can be evaluated in animal model systems, which is predictive of efficacy in human disease. For example, animal models for evaluating the efficacy of glucose uptake include animal models for diabetes or other related animal models in which glucose infusion rates can be measured. Animal models for evaluati...
Embodiment 1
[0267] Example 1: General Procedure for the Preparation of 4-Hydroxyisoleucine Analogs
[0268] A) General Experimental Procedures
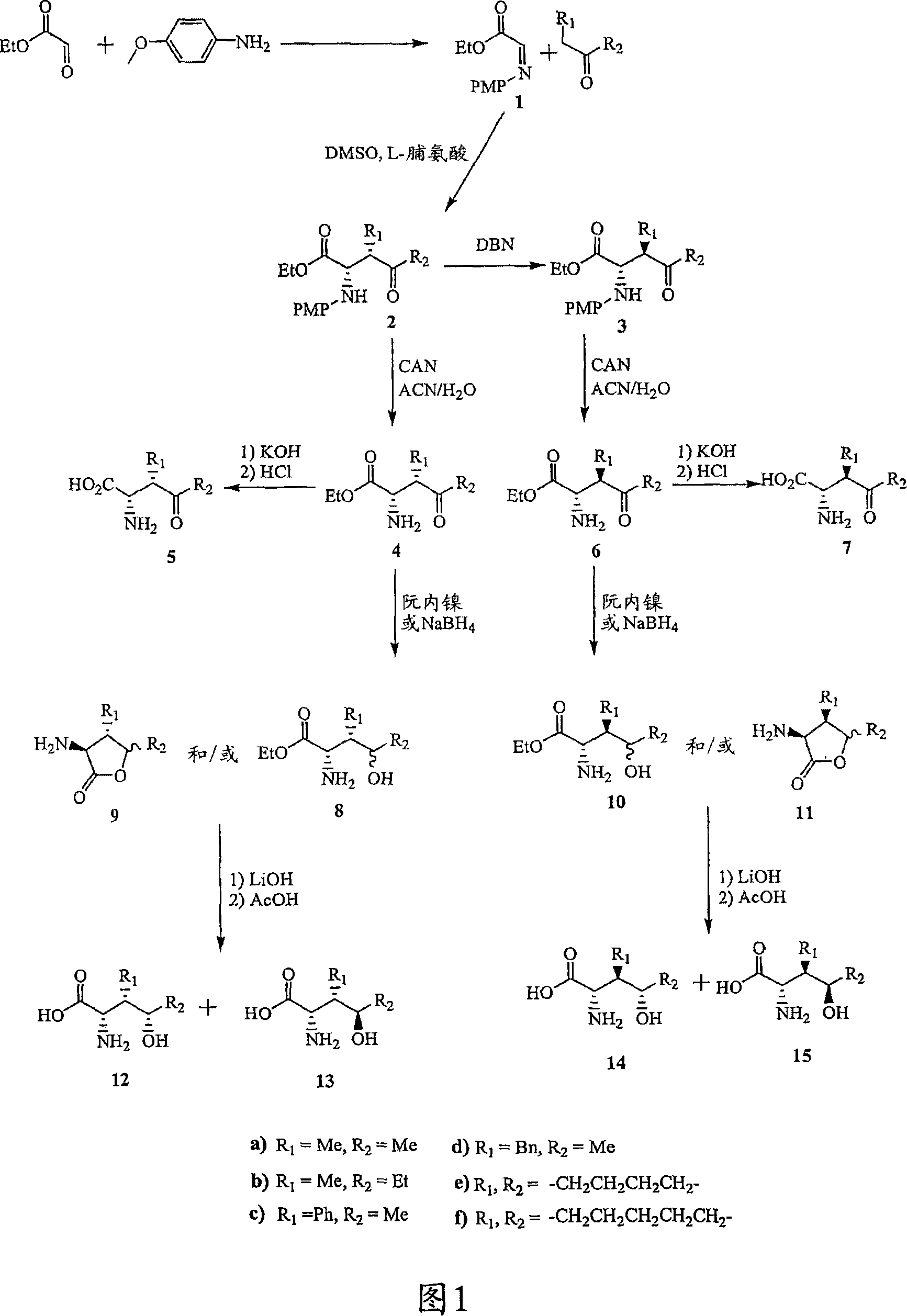
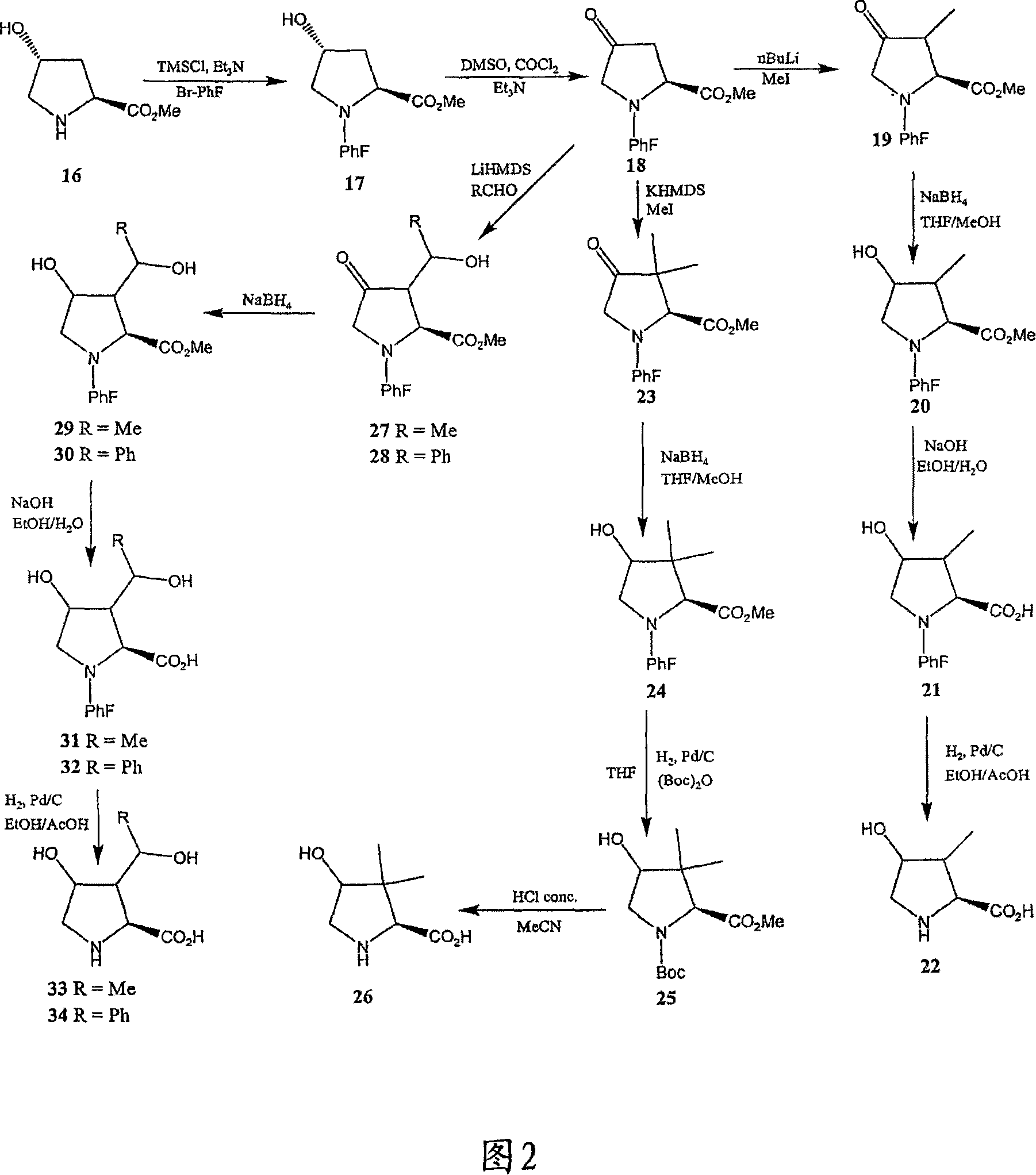
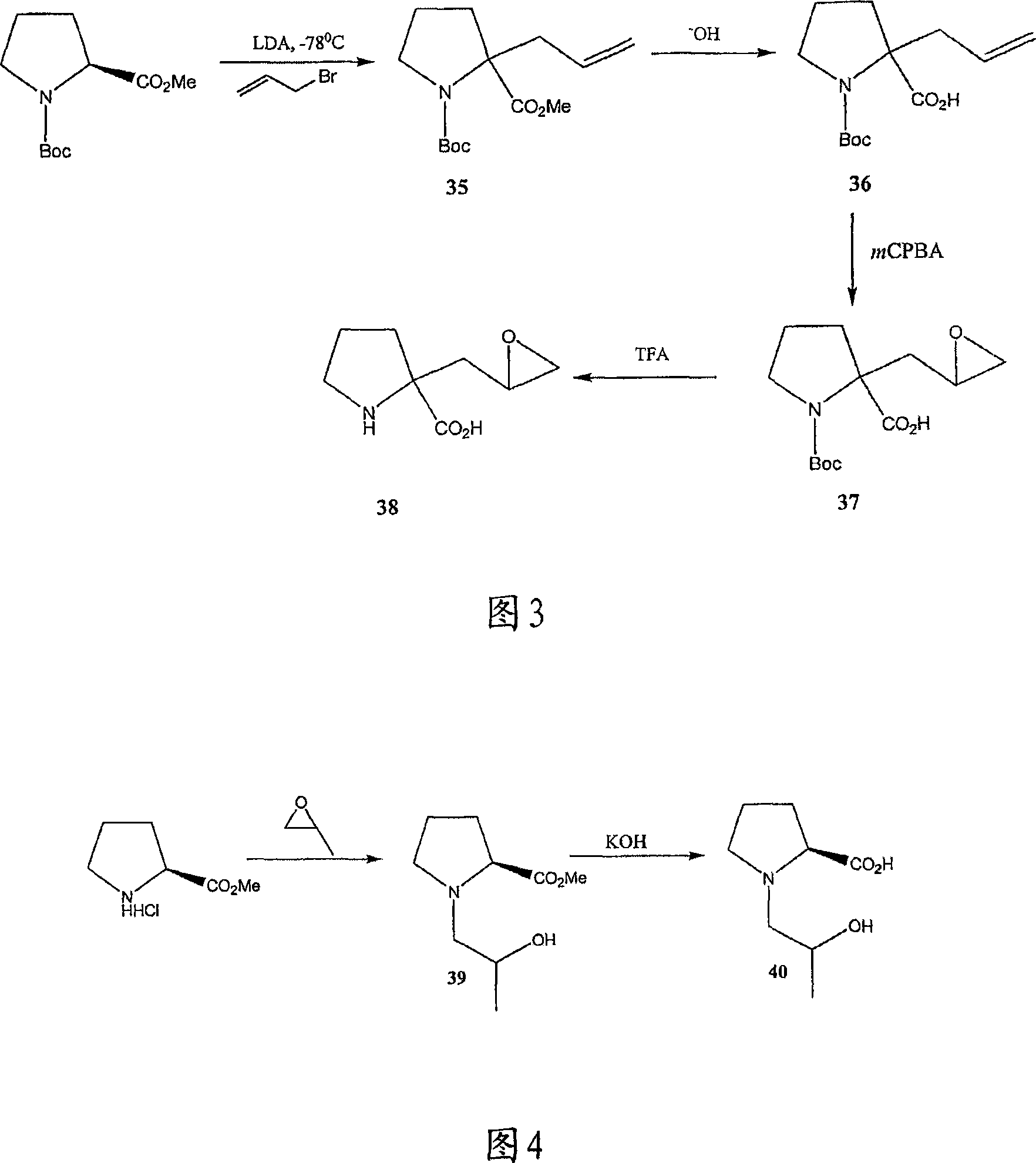
[0269] Referring to Figures 1 to 14, there are shown synthetic schemes for exemplary linear and cyclic analogs of 4-hydroxyisoleucine.
[0270] Figure 1 shows the synthesis of various 4-hydroxyisoleucine analogs with SSS, SSR, SRS and SRR configurations. Imine intermediate 1 was prepared from p-anisidine and ethyl glyoxylate (Cordova et al., J. Am. Chem. Soc. 124:1842-43, 2002). Reaction of imine 1 with the appropriate ketone in the presence of L-proline (as catalyst) yields the 2S,3S isomer (2). Epimerization at C-3 is achieved using a base such as 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) to give the 2S,3R isomer (3 ). The (2S, 3S, 4S), (2S, 3S, 4R), (2S, 3R, 4S) and (2S, 3R, 4R) analogs of 4-hydroxyisoleucine were obtained from 2 or 3, respectively, as follows:
[0271] Deprotection of the amine moiety of 2 (removal of the p-methoxyphenyl gr...
Embodiment 2
[0649] Example 2: Glucose uptake by 4-hydroxyisoleucine analogues in differentiated 3T3-L1 adipocytes stimulation
[0650] The analogs selected according to the present invention were tested for differentiating 3T3-L1 adipocytes 3 The role of H-deoxyglucose uptake. Briefly, 3T3-L1 adipocytes (ATCC; CI-173) were cultured in 12-well tissue culture plates for three days to reach confluence (Lakshmanan et al., Diabetes Mellitus: Methods and Protocols), Saire Ozena, Ed. , Humana Press Inc., Tonowa, New Jersey 97-103, 2003). The culture medium was removed and replaced with differentiation medium (Green and Meuth, Cell 3: 127-133, 1974; Madsen et al., Biochem. J. 375: 539-549, 2003), and then the cells were cultured for another 9 days. Differentiation status was determined by visual inspection. Cells were starved for 5 hours by replacing the differentiation medium with medium lacking fetal bovine serum. During the last 30 minutes of the starvation period, cells were exposed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com