Substituted oxazole derivatives and their use as tyrosine kinase inhibitors
A technology of oxazole and substituents, applied in the field of substituted oxazole derivatives, can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
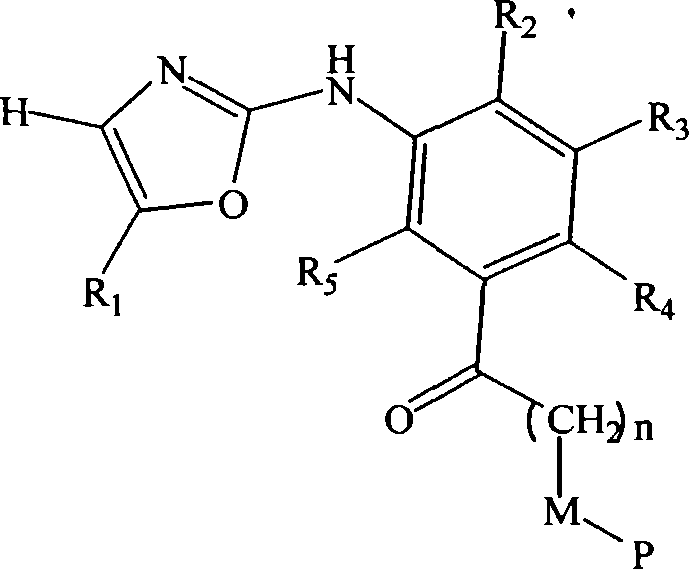
[0137] 017: 1-[4-Methyl-3-(5-pyridin-4-yl-oxazol-2-ylamino)-phenyl]-3-phenyl-propan-1-one
[0138]
[0139] m.p.=138℃
[0140] 018: 4-[2-(5-Acetyl-2-methyl-phenylamino)-oxazol-5-yl]-benzonitrile
[0141]
[0142] m.p.=240℃
[0143] 019: 4-(2-{5-[3-(4-fluoro-phenyl)-propionyl]-2-methyl-phenylamino}-oxazol-5-yl)-benzonitrile
[0144]
[0145] m.p.=175℃
[0146] 020: 4-{2-[2-Methyl-5-(3-phenyl-propionyl)-phenylamino]-oxazol-5-yl}-benzonitrile
[0147]
[0148] m.p.=138℃
[0149] 021: 4-(2-{5-[3-(3-fluoro-phenyl)-propionyl]-2-methyl-phenylamino}-oxazol-5-yl)-benzonitrile
[0150]
[0151] m.p.=157℃
[0152] 022: 4-[2-(5-Acetyl-2-methyl-phenylamino)-oxazol-5-yl]-benzamide
[0153]
[0154] m.p.>260℃
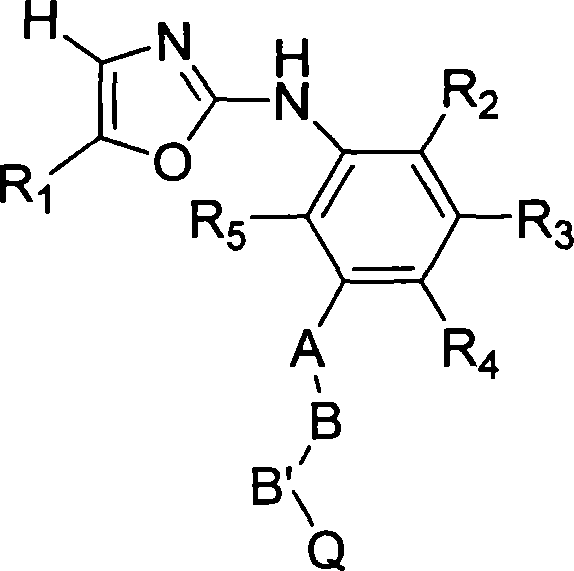
[0155] Among the specific compounds of the general formula I, the compounds referred to in the present invention are shown in the following general formula IV:
[0156]
[0157] Pass IV
[0158] G is oxygen, sulfur, N(R11) or (CH2) n , where n is 1 or 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com