Crystalline forms of pitavastatin calcium
A technology of crystallization and calcium salts, applied in metabolic diseases, cardiovascular system diseases, organic chemistry, etc., can solve the problem of not giving the precise process and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
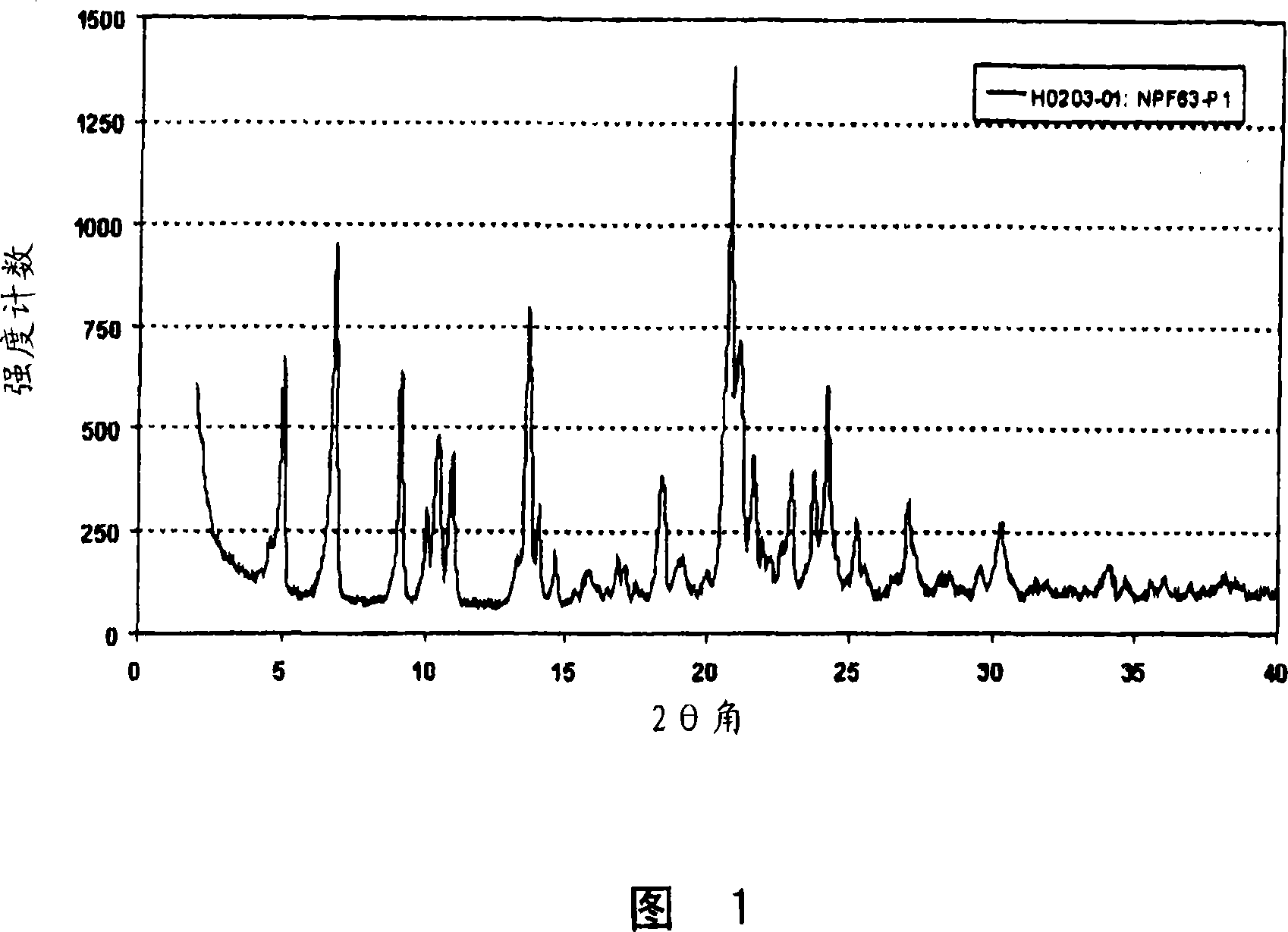
[0057] Example 1: Preparation of Form A
[0058] 4.15gr of (3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxy-6(E)-heptanoic acid The tert-butyl ester (pitavastatin tert-butyl ester) was suspended in 52 ml of a mixture of methyl tert-butyl ether and methanol (10:3). To this mixture was added 2.17 ml of 4M aqueous NaOH solution, and the obtained yellow solution was stirred at 50°C for 2.5 hours. The reaction mixture was cooled to room temperature, then 50 ml of water were added and stirred for a further hour. The aqueous phase was separated and extracted once with 20 ml of methyl tert-butyl ether. Add 0.58gr of CaCl to this aqueous solution within 1 hour 2 Solution in 80ml of water. The obtained suspension was stirred at room temperature for about 16 hours. The suspension was filtered and the solid obtained was dried at 40° C. and 50 mbar for about 16 hours. The product obtained was Form A, which was characterized by the X-ray powder diffraction patt...
Embodiment 2
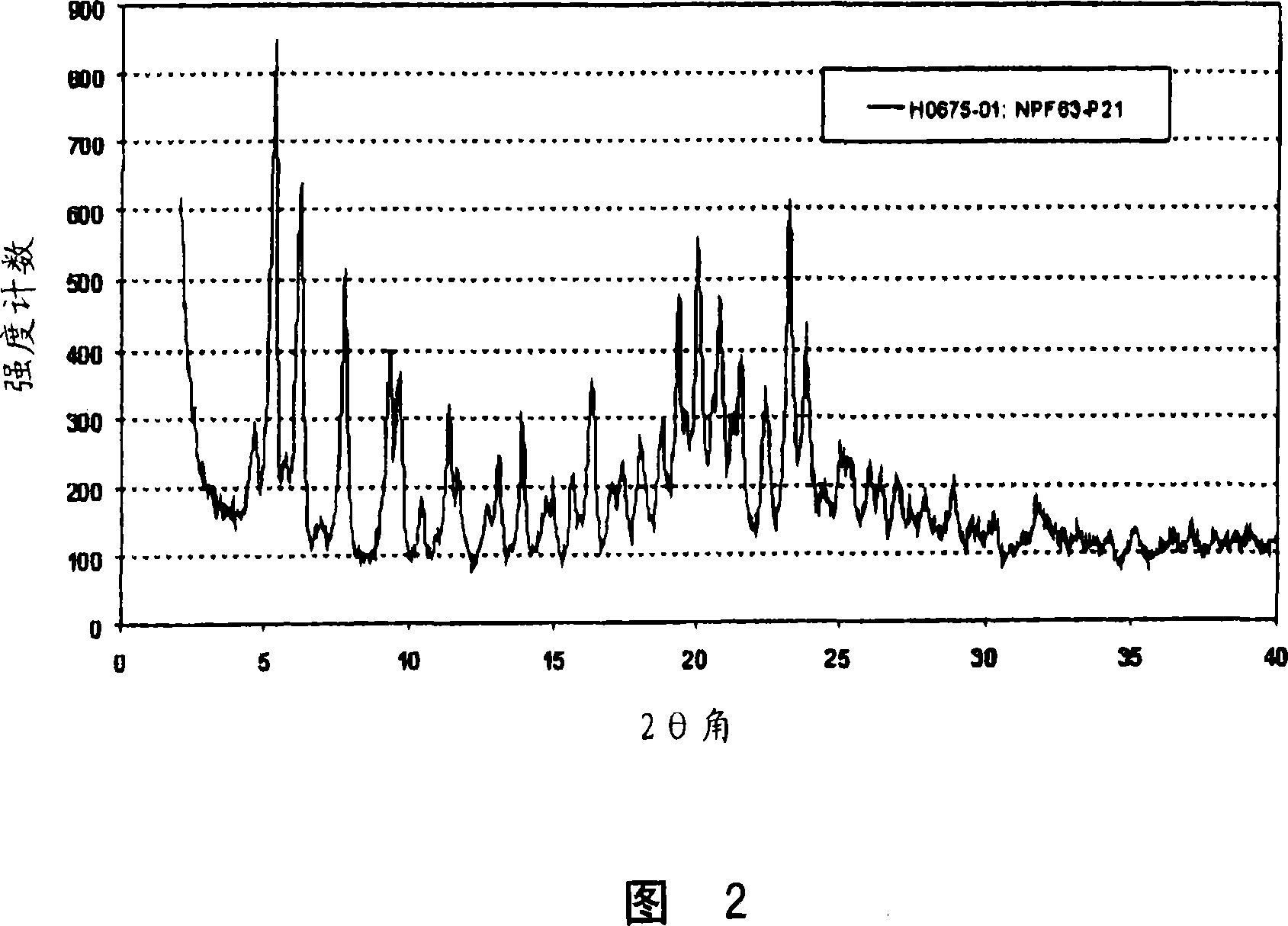
[0059] Example 2: Preparation of Form B
[0060] 100 mg of pitavastatin calcium Form A was suspended in 2 ml of water and stirred at room temperature for 30 min, followed by the addition of 2 ml of ethanol and stirring for an additional 18 hours. The suspension was filtered and air dried to afford 36 mg of Form B. The obtained Form B is characterized by the X-ray powder diffraction pattern shown in FIG. 2 . Further characterization of the obtained Form B by thermogravimetric analysis combined with FT-IR spectroscopy revealed a water content of about 10%.
Embodiment 3
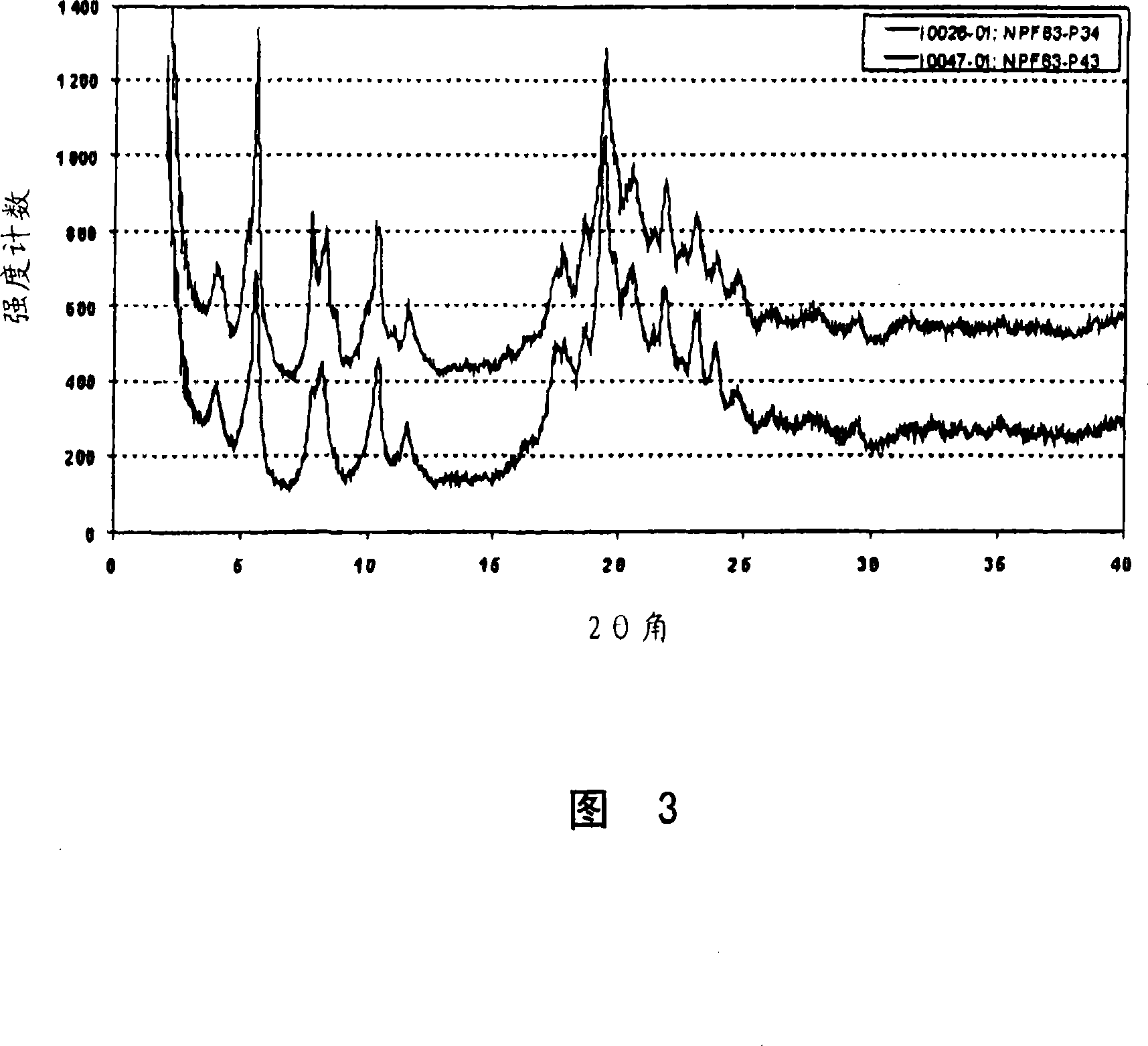
[0061] Example 3: Preparation of Form C
[0062] Suspend 62 mg of pitavastatin calcium Form A in 2 ml of isopropanol containing 5% water. This suspension was heated to 60°C, which resulted in almost complete dissolution of Form A, and cooled to room temperature again. The suspension was stirred at this temperature for 66 hours. The obtained suspension was filtered, washed once with some isopropanol containing 5% water and dried in air. The obtained Form C is characterized by the X-ray powder diffraction pattern shown in FIG. 3 . Further characterization of the obtained Form C by thermogravimetric analysis combined with FT-IR spectroscopy revealed that the sample contained about 6.3% isopropanol and a small amount of water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com