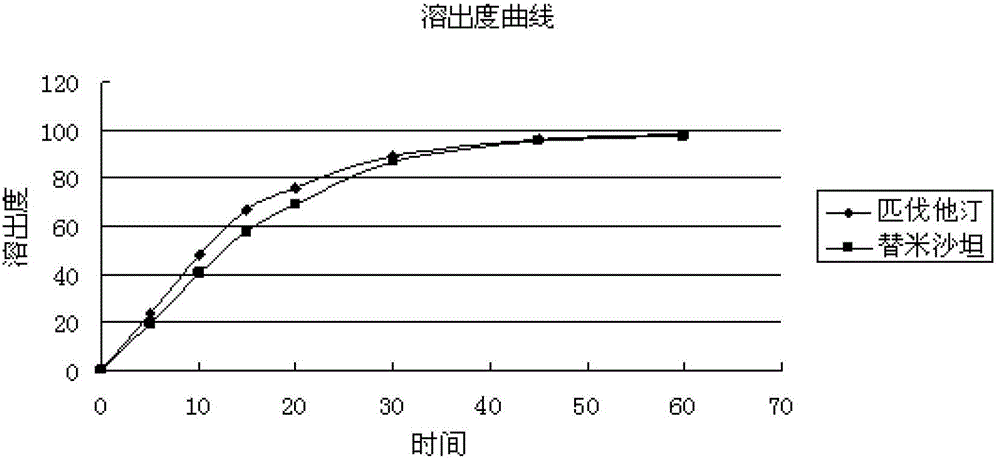
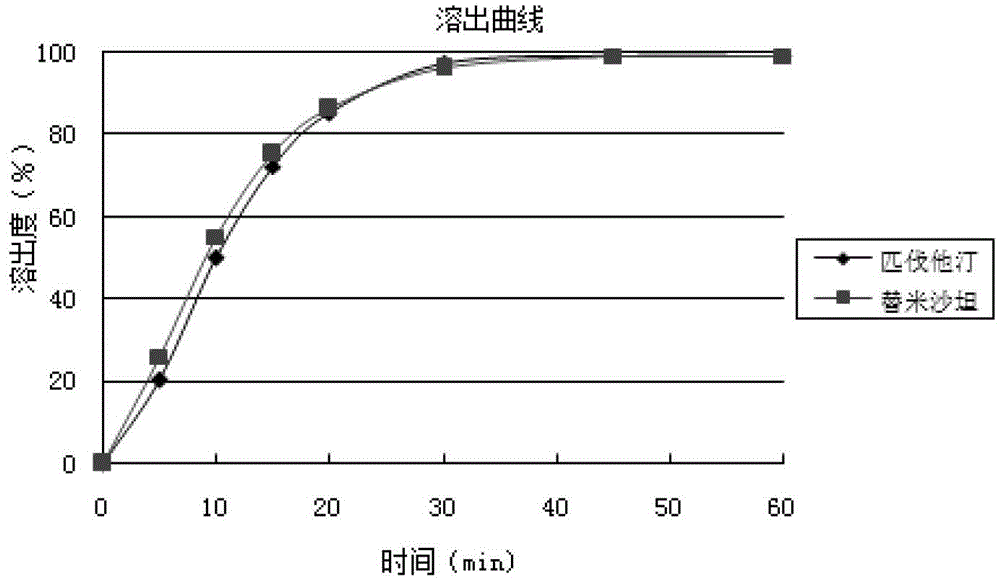
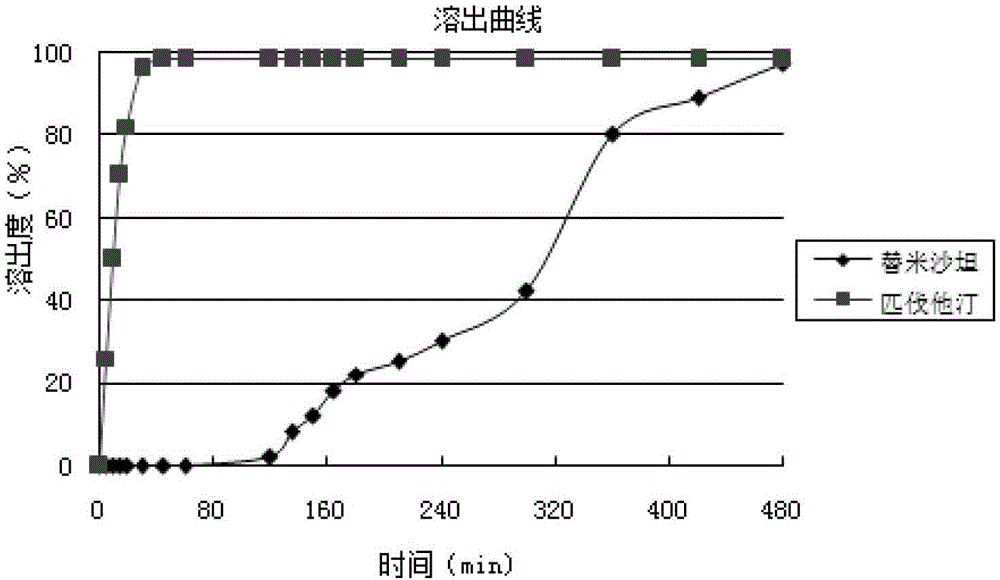
Medicinal composition containing telmisartan and pitavastatin
A technology of telmisartan and pitavastatin, which is applied in the field of joint application of antihypertensive and lipid-lowering drugs, can solve the problems of reducing the sensitivity of antihypertensive drugs and increasing the difficulty of clinical antihypertensive treatment, and achieves the protection of endothelial cells and antihypertensive drugs. Effects of oxidation, low potential for drug interaction, and low incidence of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0057] Experimental Example 1: Blood Pressure and Lipid Lowering Experiment
[0058] 1. Experimental animals:
[0059] Spontaneously hypertensive rats (SHR), raised at SPF level, male, aged 11 weeks.
[0060] WHY rats, SPF grade, male, 8 weeks old.
[0061] 2. Experimental grouping
[0062] Take 10 WHY male rats as the vehicle control group, and 90 SHR male rats were randomly divided into 9 groups according to blood pressure. The specific groups and dosages are shown in Table 1:
[0063] Table 1: Grouping, animal sex, quantity and dosage
[0064] group
gender
quantity
Dose (mg / kg)
Vehicle control group
the male
10
Equal volume of solvent
Model control group
the male
10
Equal volume of solvent
Group 0 (telmisartan / pitavastatin calcium)
the male
10
3.6mg / kg+0.18mg / kg
Group 1 (telmisartan sodium salt / pitavastatin calcium salt)
the male
10
3.6mg / kg+0.18mg / kg
Group 2 (telmis...
experiment example 2
[0092] Experimental Example 2: Optimal Experiment of the Dosage Range of the Combination of Telmisartan and Pitavastatin Calcium
[0093] Taking Telmisartan and Pitavastatin Calcium as an example, the dose ratio was tested. The experimental methods and results are as follows:
[0094] 1. Experimental animals: the same as Experimental Example 1.
[0095] 2. Experimental grouping
[0096] 10 healthy WKY rats were taken as the vehicle control group, and 110 SHR rats were randomly divided into 11 groups according to blood pressure, of which groups 1-10 were composed of telmisartan and pitavastatin calcium according to different ratios. For specific groups, see Table 4:
[0097] Table 4: Grouping, animal sex, quantity and dosage
[0098]
[0099] Note: The human dose is converted based on the rat dose through the body surface area
[0100] 3. Experimental method: same as experimental example 1.
[0101] 4. Detection index: same as Experiment 1.
[0102] 5. Statistical meth...
experiment example 3
[0128] Experimental Example 3: Study on the Synergistic Protective Effect on Cardiovascular System and Kidney
[0129] Taking telmisartan and pitavastatin calcium as an example, the effect experiment on cardiovascular effects was carried out. The experimental methods and results are as follows:
[0130] 1. Experimental animals: same as Experimental Example 1;
[0131] 2. Experimental grouping and methods:
[0132] Experimental group:
[0133] Ten healthy WKY male rats were taken as the vehicle control group, and 40 SHR male rats were randomly divided into 4 groups according to blood pressure. The specific groups are shown in Table 7:
[0134] Table 7: Grouping, animal sex, quantity and dosage
[0135] group
gender
quantity
Dose (mg / kg)
Vehicle control group
the male
10
-
[0136] Model control group
the male
10
-
Telmisartan group
the male
10
3.6
pitavastatin calcium group
t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com