Substituted cinnamic acid derivatives containing amine substituent group and expression purification cytotoxicity thereof
A technology of tumor cells and substituents, applied in the direction of anti-tumor drugs, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as unsatisfactory, low selectivity, and malignant killing of normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
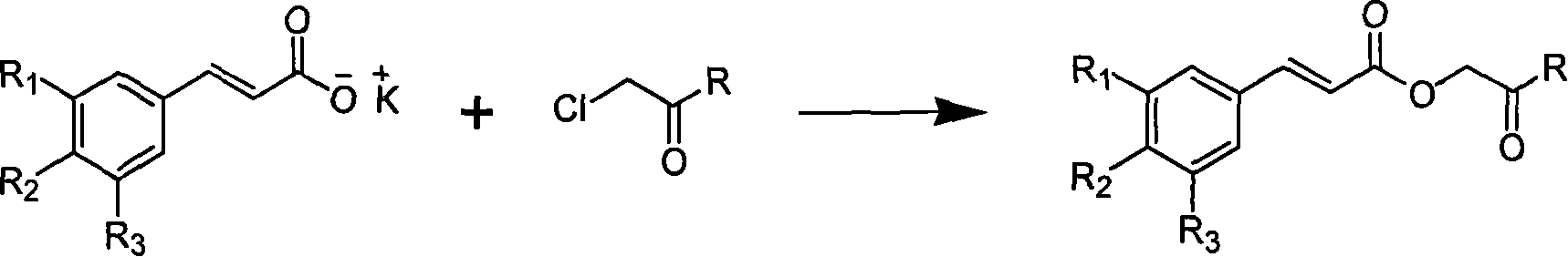
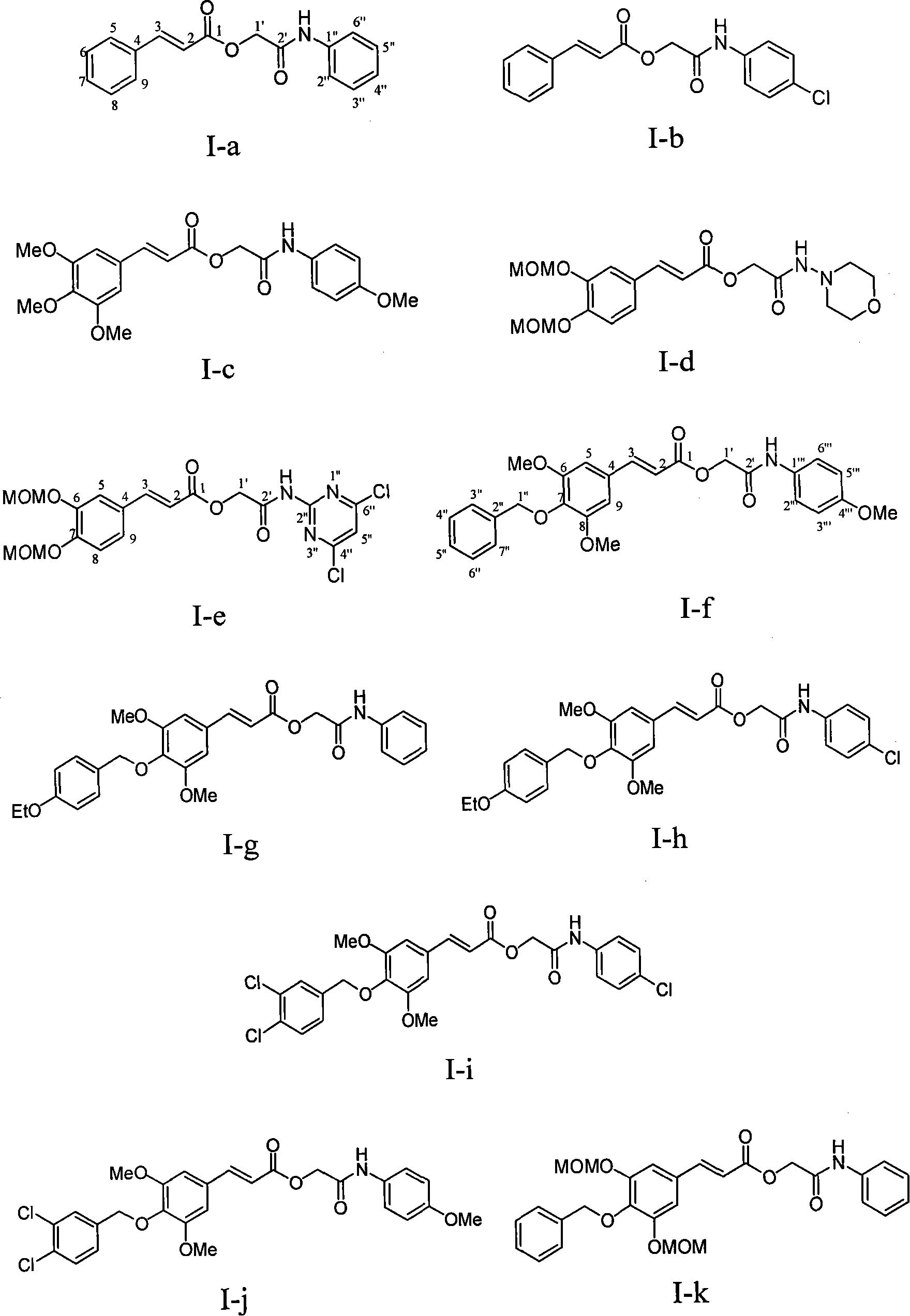
[0030] Example 1 : the preparation of compound I-a i.e. 2'-aniline-2'-oxoethyl cinnamate (2E)
[0031]
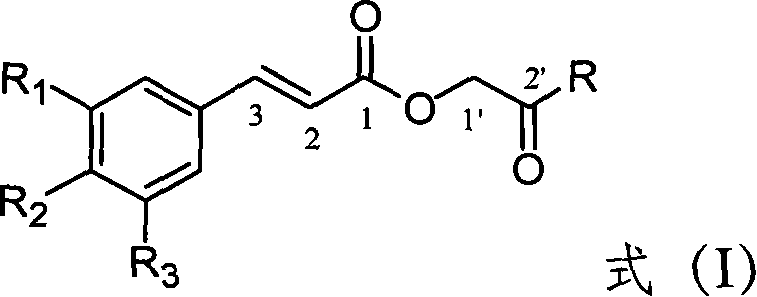
[0032] This example relates to a general synthesis method of a class of substituted cinnamic acid derivatives containing amide substituents represented by formula (I) with cytotoxic activity. It specifically relates to the preparation of compound 2'-aniline-2'-oxoethyl cinnamate (2E). Dissolve chloroacetylaniline (85 mg, 0.50 mmol, prepared by reacting chloroacetyl chloride with aniline) in 10 ml of DMF, add potassium iodide (91 mg, 0.55 mmol) and stir at 40°C for 20 minutes, then add cinnamon Acetate potassium salt (108 mg, 0.58 mmol), after reflux for 4 hours, concentrated under reduced pressure to remove DMF, the obtained crude product was extracted with ethyl acetate-water distribution, the ethyl acetate layer was washed with water until neutral, saturated sodium chloride solution Wash with water and dry overnight over anhydrous sodium sulfate. The obtained filtra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com