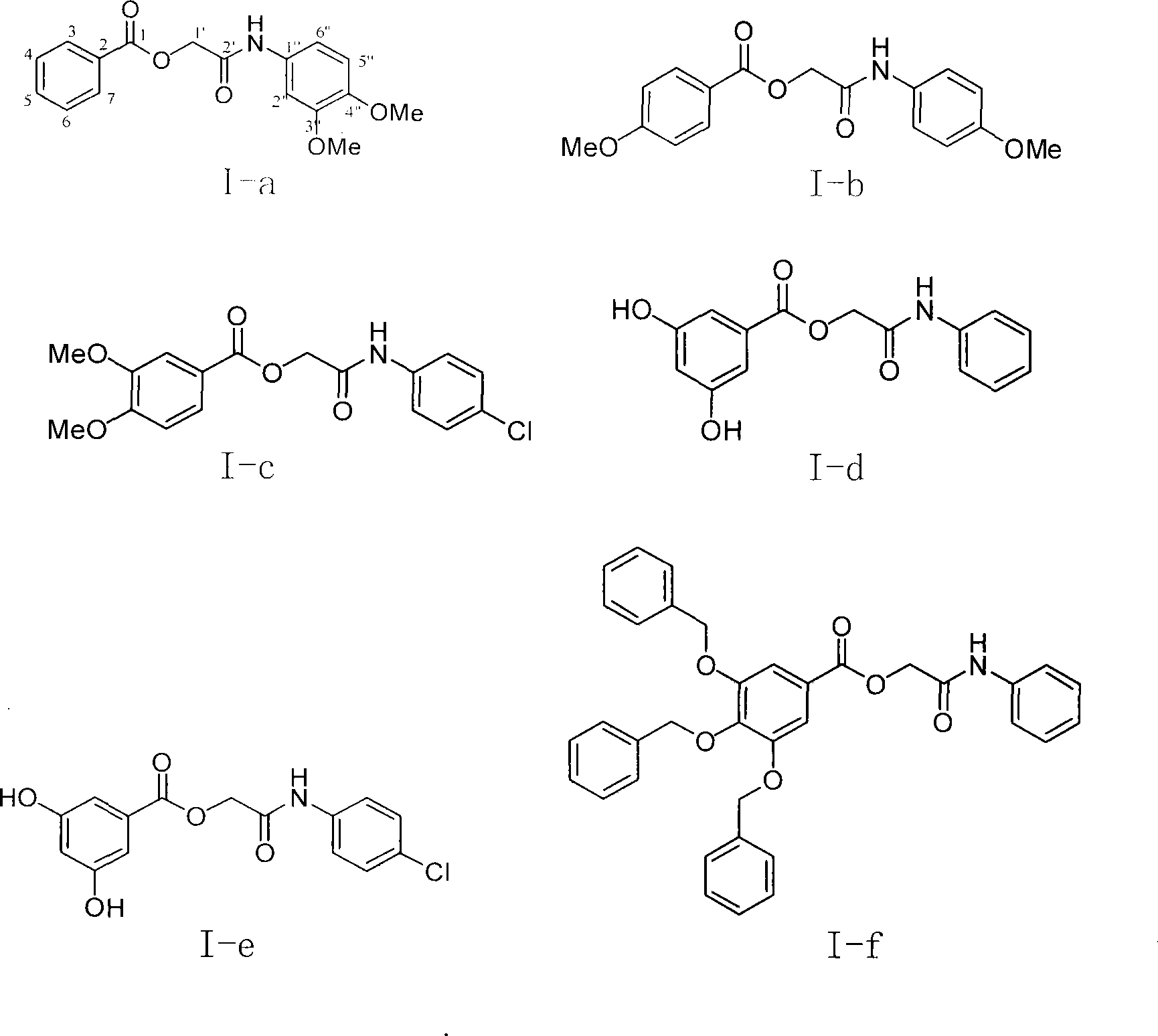
Substituted benzoic acid nitrogen-containing derivatives and antineoplastic usage thereof
A hydroxybenzoate and tumor technology, applied in the direction of anti-tumor drugs, drug combinations, ester active ingredients, etc., can solve the problems of limiting the universal applicability of drugs, unsatisfactory, malignant killing of normal cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
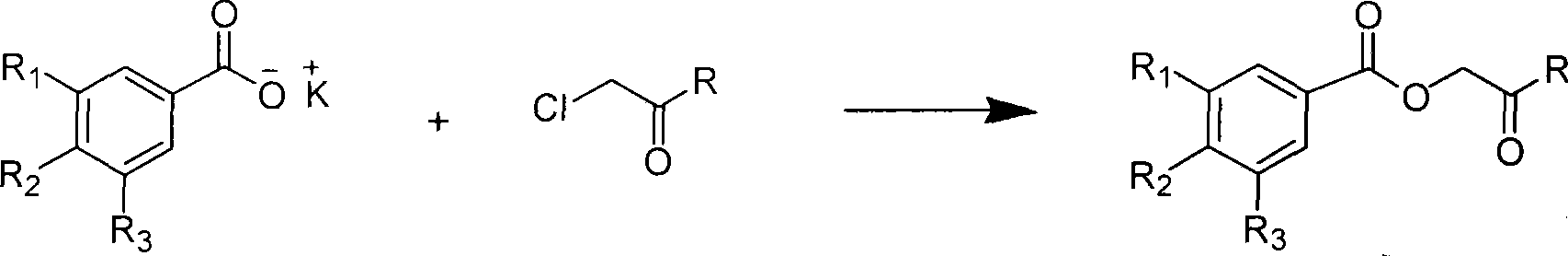
Method used
Image
Examples
Embodiment 1
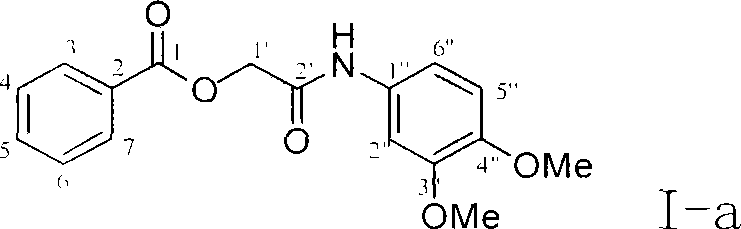
[0037] Example 1 : the preparation of compound I-a i.e. 2 '-(3 ", 4 "-dimethoxyaniline)-2'-oxoethyl-benzoic acid ester
[0038]
[0039] This example relates to a general synthesis method of a class of substituted acetylarylamine benzoate derivatives shown in formula (I) with cytotoxic activity. It specifically relates to the preparation of compound 2'-(3",4"-dimethoxyaniline)-2'-oxoethyl-benzoate. Dissolve chloroacetyl 3,4-dimethoxyaniline (78 mg) in 10 ml of DMF, add potassium iodide (90 mg) and stir at 40°C for 20 minutes, then add potassium benzoate (106 mg), and reflux After 4 hours, the DMF was removed by concentration under reduced pressure. The obtained crude product was partitioned and extracted with ethyl acetate-water, and the ethyl acetate layer was washed with water until neutral, washed with saturated sodium chloride solution, and dried overnight with anhydrous sodium sulfate. The obtained filtrate was filtered and concentrated, and purified by column chroma...
Embodiment 2
[0057] Pharmacological Example 2 : Cytotoxic activity of compound I-f on leukemia (HL-60) tumor cells
[0058] Leukemia (HL-60) tumor cells were cultured with RPMI 1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin. cells in 5×10 per well 3 concentration into a 96-well plate at 37 °C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0059] Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared dimethyl sulfoxide solution of compound I-f was added to each well in a concentration gradient, so that the final concentrations of the compounds in the wells were 100 μg / ml, 33.3 μg / ml, and 11.1 μg / ml and 3.7 μg / ml. After 72 hours, add 10 microliters of MTT (5 mg / ml) in phosphate buffer, continue to incubate at 37°C for 4 hours, centrifuge for 5 minutes to remove unconverted MTT, and add 200 microliters of dimethyl sulfide to each well. The sulfone was used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com