Formulation for a viptadil
A technology of pharmaceutical preparations and preservatives, applied in the field of pharmaceutical preparations of alendidil and its derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
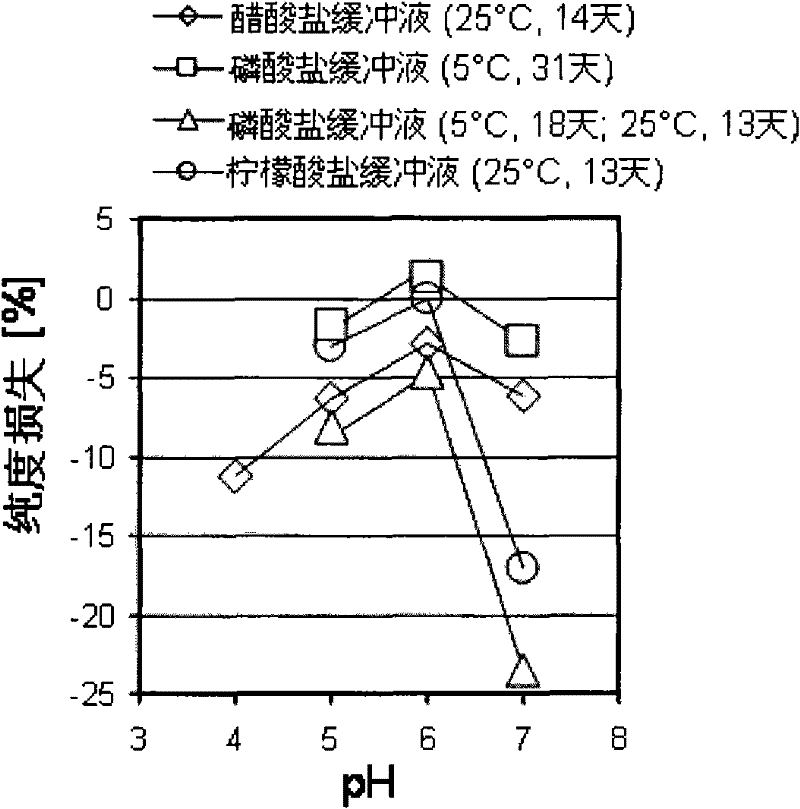
[0112] The stability of alendidil was determined over a pH range of 5 to 7 and at the temperatures and lengths of time shown in Tables 1-4 below.
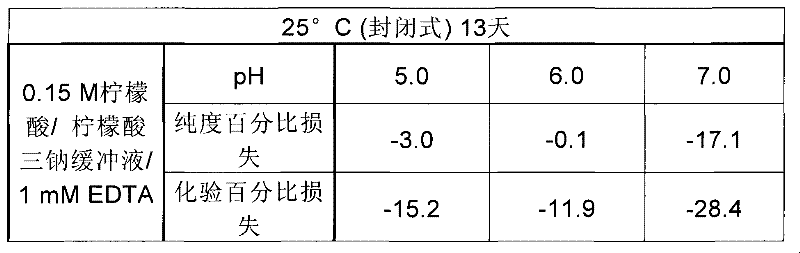
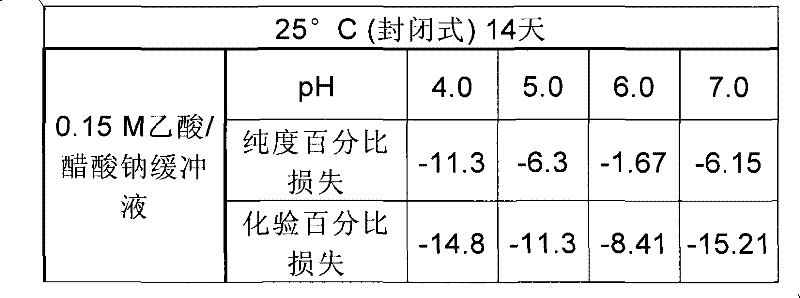
[0113] pH values for purity and assay are shown in Table 1 for peptides in 150 mM citrate buffer and 1 mM EDTA, in Table 2 for peptides in 150 mM acetate buffer, and in Table 3 for peptides in 150 mM phosphate buffer and 4.
[0114] Table 1
[0115]
[0116] Table 2
[0117]
[0118] table 3
[0119]
[0120]
[0121] Table 4
[0122]
[0123] From these tables it can be seen that alendidil is most stable at about pH 6. figure 1 The purity loss data in the table above were summarized to more clearly show the increased stability of alendidil at about pH 6.
Embodiment 2
[0125] This example describes the liquid formulation of alendidil using citrate buffer or 0.9% sodium chloride without buffer components. The pH of the sodium chloride solution is between 5.3 and 5.8. A solution with a pH value of approximately 6 was shown to increase the stability of alentidil (see Example 1).
[0126] Another important characteristic of the formulation is the amount of alendidil in solution. Surprisingly, the inventors found that alentidil in formulations comprising alentidil in amounts ranging from 0.0066% to 0.2% was more stable than formulations comprising alentidil in amounts less than 0.0066%, as measured by the percentage loss of the test (See Table 5). The purity of the samples did not show any significant change at different concentrations of alendidil. When the concentration of alendidil increased from 0.033 mg / Ml to 0.066 mg / mL, the shelf life increased approximately 3-fold.
[0127] table 5
[0128] Altidel concentration 0.033...
Embodiment 3
[0131] Based on the finding that alendidil is most stable at a pH of about 6 and an amount of alendidil of about 0.0066%, the following formulations were developed:
[0132] Formulation A
[0133] Element Weight % (w / v) Atendil 0.0066-0.5 Sodium chloride 0.9 water for injection 100mL
[0134] In formulation B, mannitol was added to the formulation to increase the stability of alentidil.
[0135] Formulation B
[0136] Element Weight % (w / v) Atendil 0.0066-1.0 Mannitol 4.0 Sodium chloride 0.16 water for injection 100mL
[0137] Formulation C
[0138] Element Weight % (w / v) Atendil 0.001-1.0 Citrate buffer (50mM, pH 5.8) citric acid 0.227 Trisodium citrate dihydrate 1.12 Mannitol 4.0 water for injection 100mL
[0139] Preparation D
[0140] Element Weight % (w / v) Atendil 0.001-1.0 Citrate buffer (120mM,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com