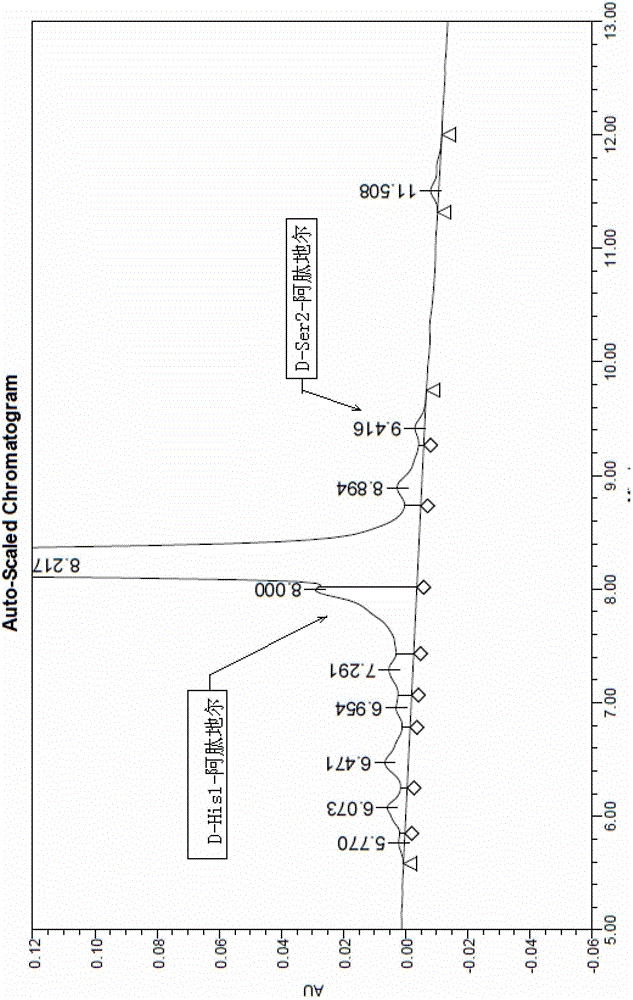
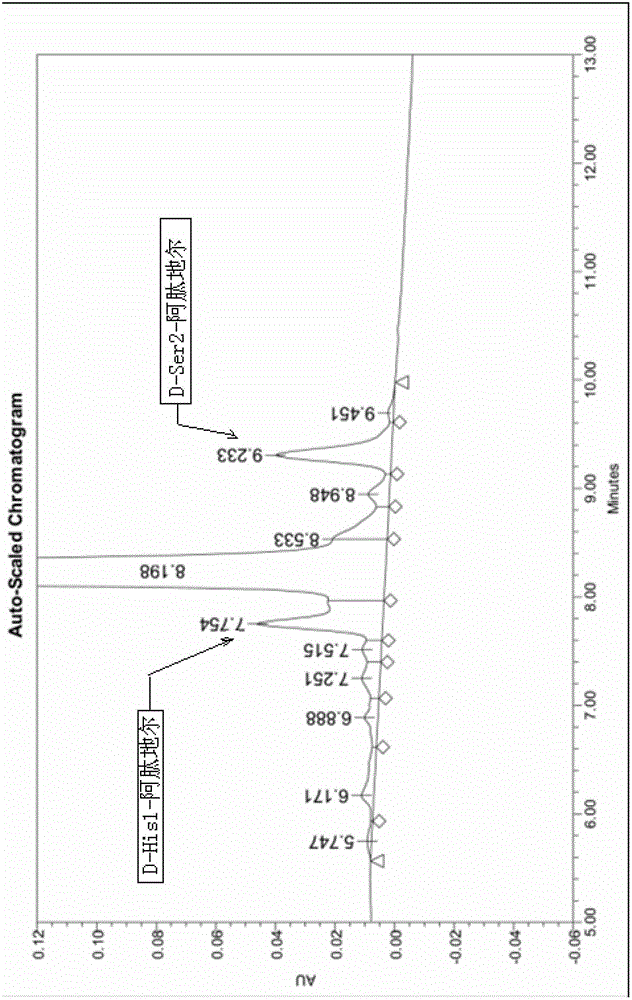
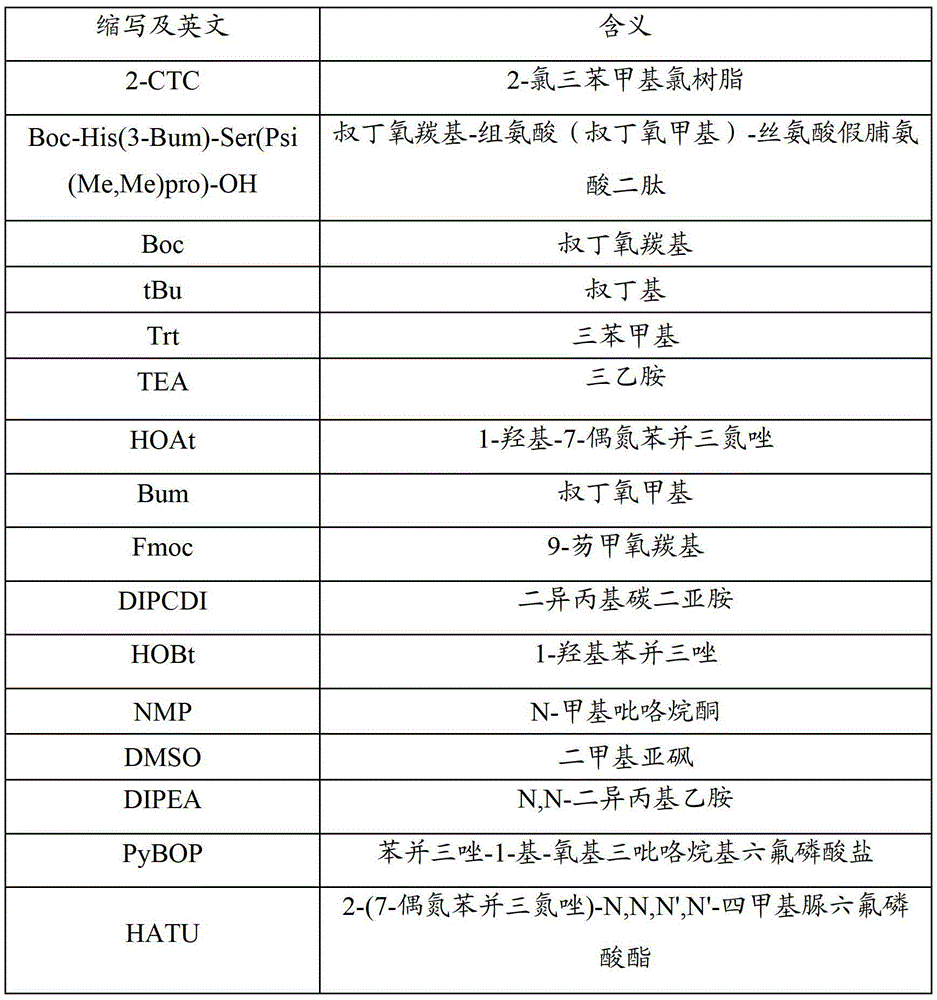
Method for synthetizing aviptadil
A technology of solid-phase synthesis of alendidil, which is applied in the field of pharmaceutical synthesis, and can solve the problems of many impurities, the yield and purity cannot reach a high level, and the impurities are difficult to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Synthesis of Polypeptide Fragment Resin I
[0054] Weigh 100 g (20 mmol) of Rink Amide resin with a degree of substitution of 0.2 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then add 20% piperidine / DMF (V: V) 100ml solution, deprotection for 5 and 10 minutes, after the reaction, wash the resin 6 times with DMF, and use ninhydrin to detect the color of the resin. Weigh 23.87g Fmoc-Asn(Trt)-OH (40mmol), HOBt5.94g (44mmol) and dissolve it with 80ml DMF / DCM (1:1, V:V), add 6.85ml DIPCDI (44mmol) to activate for 3 minutes under ice-water bath Finally, add it to the above-mentioned reaction column equipped with resin and react for 2 hours. The end point of the reaction is judged by ninhydrin detection (if the resin is colorless and transparent, the reaction is complete; if the resin develops color, continue to reflect for 1 hour. The same below), After the reaction, the resin was washed 3 ti...
Embodiment 2
[0057] Example 2: Synthesis of Polypeptide Fragment Resin I
[0058] Weigh 33.3g (20mmol) of Rink Amide resin with a substitution degree of 0.6mmol / g, add it to the solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then add 20% piperidine / DMF (V : V) 100ml solution, deprotection for 5 and 10 minutes, after the reaction, wash the resin 6 times with DMF, and use ninhydrin to detect the color of the resin. Weigh 23.87g Fmoc-Asn(Trt)-OH (40mmol), HOBt5.94g (44mmol) and dissolve it with 80ml DMF / DCM (1:1, V:V), add 6.85ml DIPCDI (44mmol) to activate for 3 minutes under ice-water bath Finally, add it to the above-mentioned reaction column equipped with resin and react for 2 hours. The end point of the reaction is judged by ninhydrin detection (if the resin is colorless and transparent, the reaction is complete; if the resin develops color, continue to reflect for 1 hour. The same below), After the reaction, the resin was washed 3 time...
Embodiment 3
[0061] Example 3: Synthesis of Polypeptide Fragment Resin I
[0062] Weigh 20 g (20 mmol) of Rink Amide resin with a substitution degree of 1.0 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then add 20% piperidine / DMF (V: V) 100ml solution, deprotection for 5 and 10 minutes, after the reaction, wash the resin 6 times with DMF, and use ninhydrin to detect the color of the resin. Weigh 23.87g Fmoc-Asn(Trt)-OH (40mmol), HOBt5.94g (44mmol) and dissolve it with 80ml DMF / DCM (1:1, V:V), add 6.85ml DIPCDI (44mmol) to activate for 3 minutes under ice-water bath Finally, add it to the above-mentioned reaction column equipped with resin and react for 2 hours. The end point of the reaction is judged by ninhydrin detection (if the resin is colorless and transparent, the reaction is complete; if the resin develops color, continue to reflect for 1 hour. The same below), After the reaction, the resin was washed 3 times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com