Dyestuffs and hair dye compositions
A technology of dyes and alkyls, applied in the field of hair dyeing compositions, which can solve the problems of limited number of dyes, difficulty in obtaining purple, low durability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
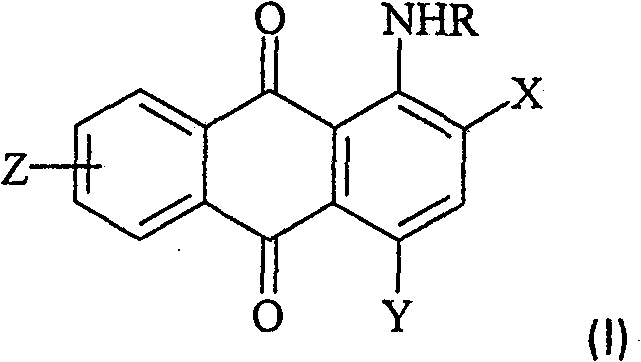
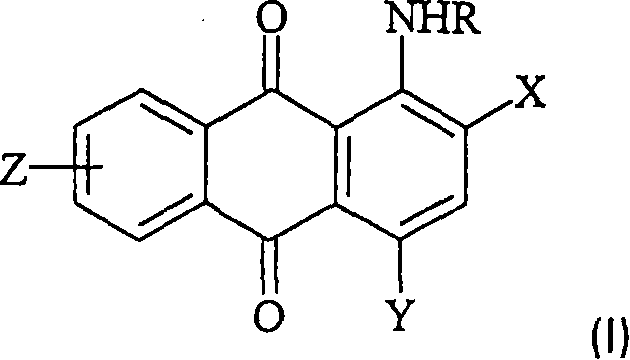
[0109] (Preparation of [3-(4,8-diamino-9,10-dioxo-9,10-dihydro-anthracene-1-ylamino)-propyl]-trimethyl-ammonium methylsulfate)
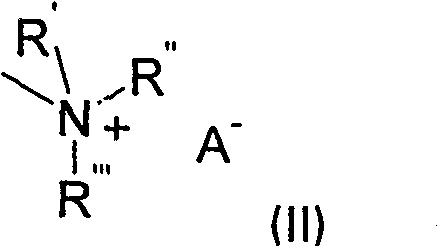
[0110] 90 parts of 3-dimethylaminopropylamine, 10.0 parts of 1,5-diamino-4-chloro-anthraquinone and 0.25 parts of copper(II) acetate were heated together under nitrogen at 110° C. for 7.5 hours. After cooling at room temperature, the mixture was poured into 500 parts of cold water. The precipitate was filtered off, washed with water and dried to yield 10.7 parts of a dark blue powder. 9.0 parts of the obtained blue dye were dispersed in 100 parts of ethanol, and treated with 3.0 parts of dimethyl sulfate, thereby achieving quaternization. The reaction mixture was stirred at room temperature for 3.5 hours. The precipitate was filtered off and washed with acetone. After drying, 10.7 parts of a dye of formula Ij are obtained. Blue dye Ij has very good water solubility. The analytical data are consistent with the assigned structure of dye Ij. 1 H N...
Embodiment 2
[0114] (Methylsulfate [3-(4-amino-3-methyl-9,10-dioxo-9,10-dihydro-anthracen-1-ylamino)-propyl]-trimethyl-ammonium) preparation of
[0115] 85 parts of 3-dimethylaminopropylamine, 15.8 parts of 1-amino-4-bromo-2-methylanthraquinone and 0.17 parts of copper(II) acetate were heated together under nitrogen at 94° C. for 2.5 hours. After cooling at room temperature, water was added until the product precipitated. The product was filtered off, washed and dried, yielding 14.0 parts of a dark purple powder. Quaternization was achieved by dispersing 10 parts of the obtained violet dye in 100 parts of chlorobenzene and treating with 2.8 parts of dimethyl sulfate. The temperature was raised to 45°C and the reaction mixture was maintained at this temperature for 3 hours. The precipitate was filtered off at 30°C and washed with acetone. After drying, 12 parts of a dye of the formula Ia are obtained. Violet dye Ia has very good water solubility. The analytical data are consistent wit...
Embodiment 3
[0120] (Methylsulfate [4-(4-amino-3-methoxy-9,10-dioxo-9,10-dihydro-anthracen-1-ylamino)-phenyl]-trimethylammonium) preparation of
[0121] 21.4 parts of 1-amino-4-bromo-9,10-dioxo-9,10-dihydro-anthracene-2-sulfonic acid were dispersed in 100 parts of water at 50°C. After that, 9.8 parts of N,N-dimethyl-p-phenylenediamine, 5.0 parts of NaHCO 3 , 2.7 parts of Na 2 CO 3 and 0.5 parts of CuCl. The reaction mixture was maintained at 50-60°C for 2 hours. After cooling at room temperature, the mixture was poured into 700 parts of 2N HCl. The precipitate was filtered off, washed with water and dried, yielding 18.8 parts of a dark blue powder. 9.4 parts of the resulting dye were added to a warm solution of anhydrous KOH in methanol at 50°C. The reaction mixture was maintained at 50°C for 6 hours. After cooling, the mixture was poured into 200 parts of water and treated with concentrated HCl until the pH value was 6.5-7.0. The precipitate was filtered off, washed with water an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com