Preventive or therapeutic agent for cerebral ischemic injury or cerebral ischemia reperfusion in stroke
A technology for cerebral ischemia-reperfusion and cerebral ischemia, which is applied in the field of prevention or treatment of cerebral ischemia or cerebral ischemia-reperfusion injury in stroke, and can solve the problems of lack of pharmacological effects on blood coagulation system and circulatory system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0033] 1. Test method
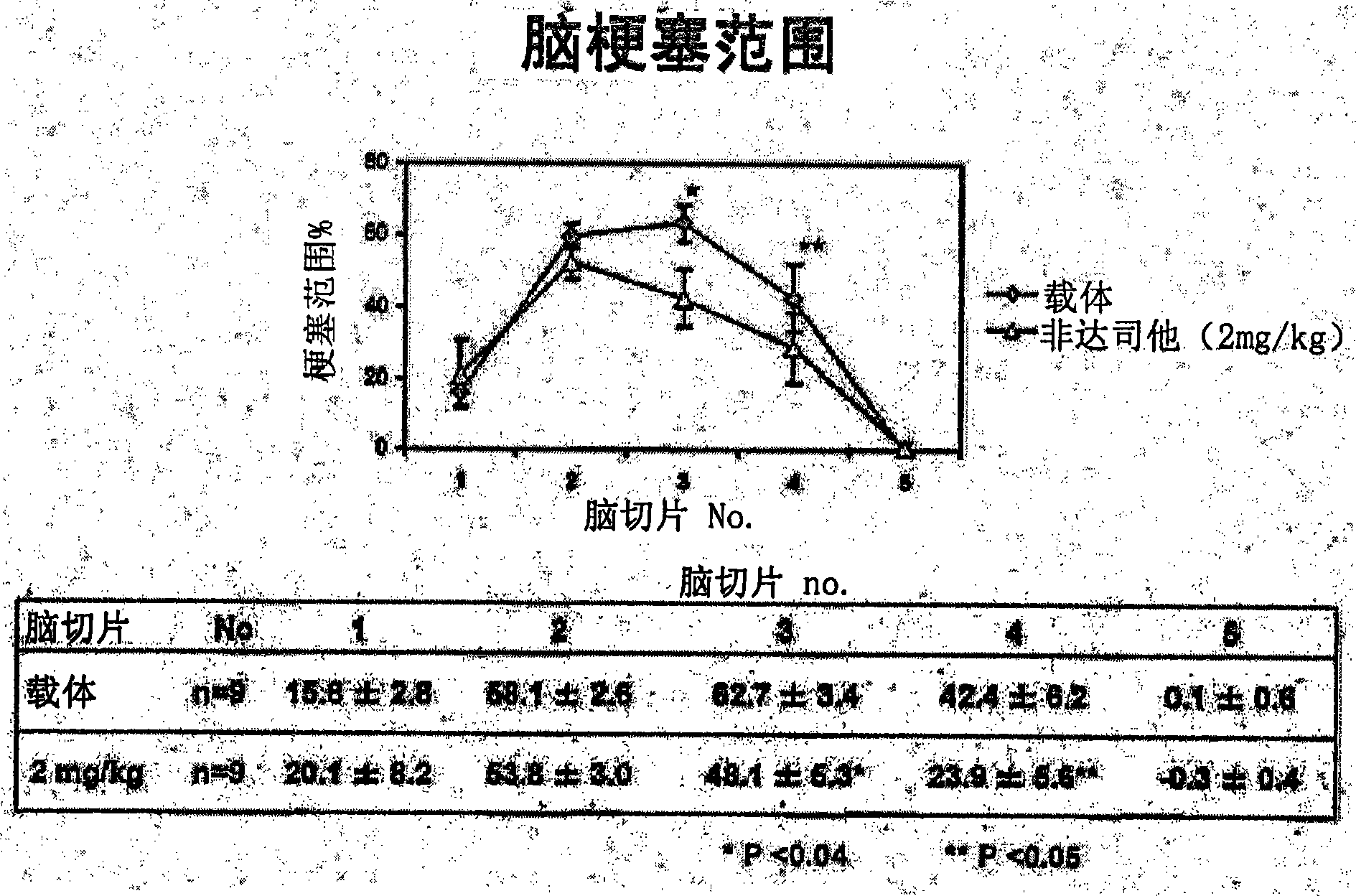
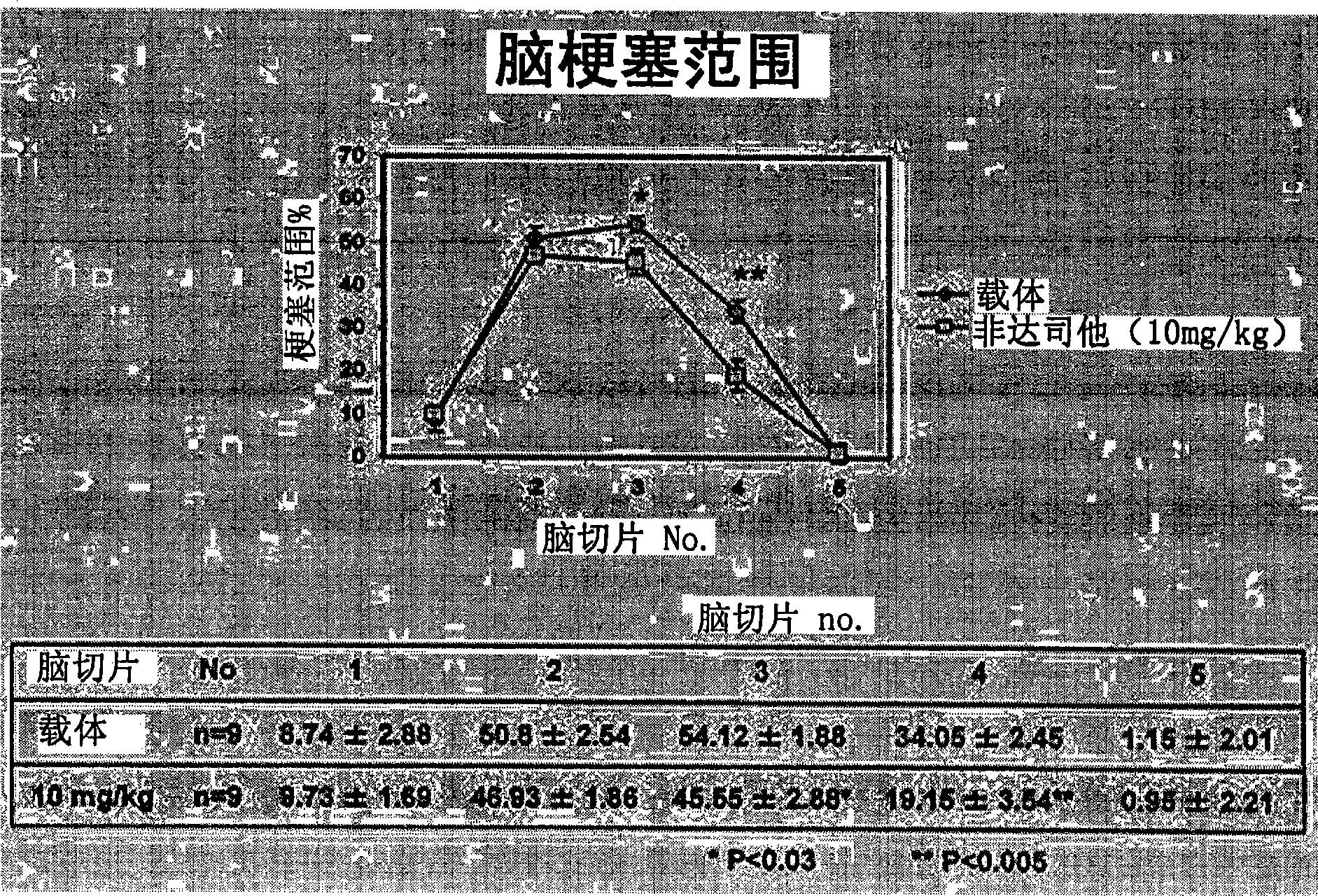
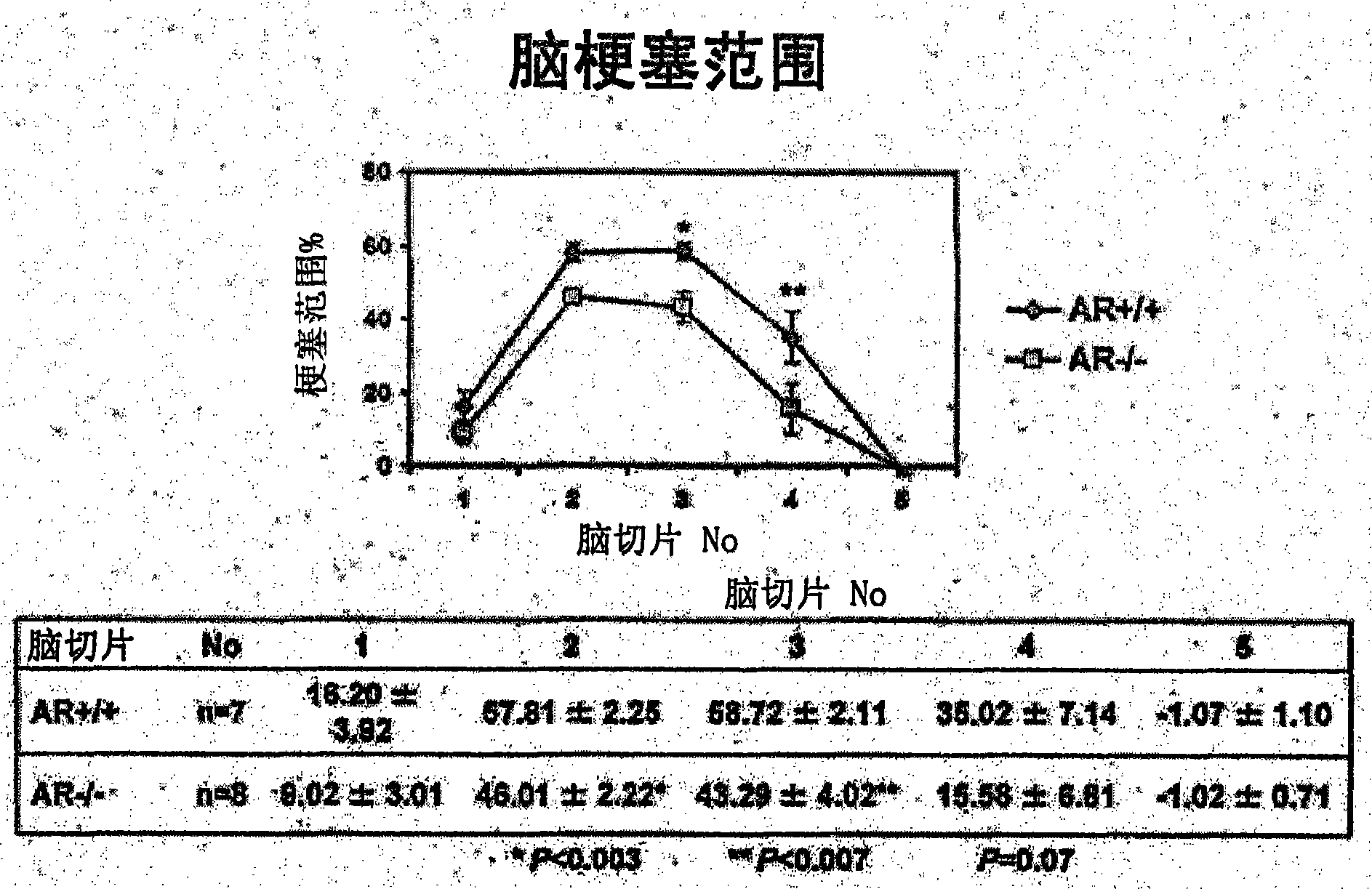
[0034] As an evaluation system, a mouse model of acute cerebral infarction, that is, a mouse model of middle cerebral artery occlusion was used. The experiment consisted of the following three experiments: Experiment 1 (control group and fidarestat administration group) to evaluate the therapeutic effect of fidarestat, experiment 2 (control group and fidarestat administration group) to evaluate the preventive effect of fidarestat He-administered group) and Experiment 3 (wild-type mouse group and AR gene-deficient mouse group) to evaluate the effect of AR.
[0035] Using mice (C57BL / 6J strain, body weight 22-28 g) under gas anesthesia, the right middle cerebral artery occlusion (MCAO) was performed by suture method for 2 hours. Thereafter the anesthesia was released and the mice were placed in an intensive care system (ThermoCare Inc) for 4-6 hours at 32°C. Neurological symptoms, cerebral infarction size and cerebral infarction volume were used as eval...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com