Waste polyester material recovery method with ion liquid as reaction medium and catalyst
A technology of ionic liquid and waste polyester, which is applied in chemical recovery, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of equipment corrosion environment, catalysts cannot be recycled, reaction conditions, harsh and other problems, and reduce the discharge of three wastes , Improve the effect of equipment corrosion and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
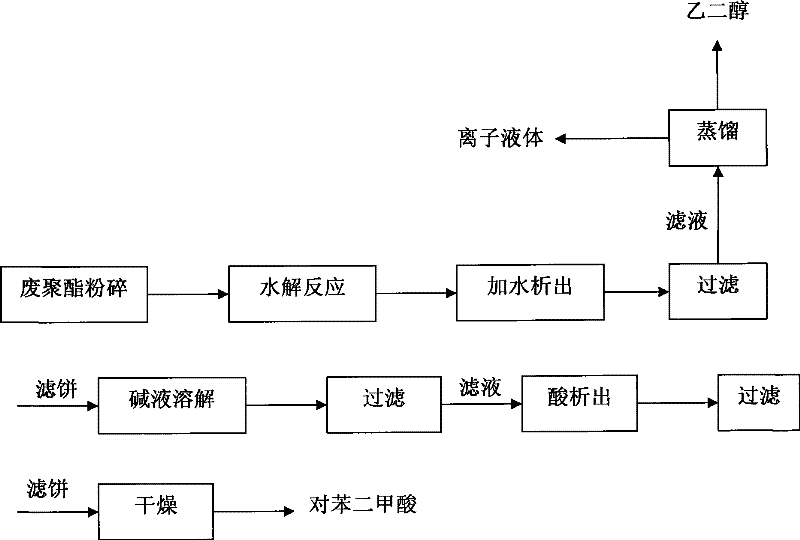
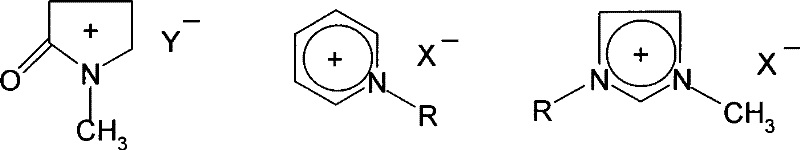
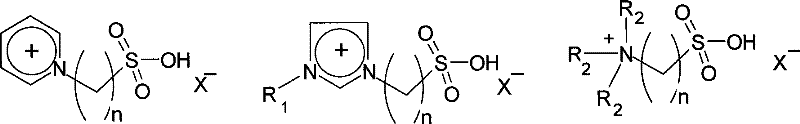
[0023] Example 1: Put 15.0g of waste PET into an autoclave, add 15.0g of water, 1.5g of 1-methyl-3-(3-sulfonic acid propyl) imidazole bisulfate, 30g of 1-butyl chloride -3-Methylimidazole, after the addition, stir and react at 190°C for 1.5h. After cooling down to room temperature, add a certain amount of water to precipitate a precipitate, filter, distill and separate the filtrate to recover ethylene glycol and ionic liquid, and dilute the filter cake with hydrogen Sodium oxide solution was dissolved, and then filtered to remove undegraded PET and other insoluble impurities, and the obtained filtrate was added with acid to precipitate, filtered and dried to obtain 11.90 g of terephthalic acid. The degradation rate of PET is 99.1%, and the yield of terephthalic acid is 91.8%.
Embodiment 2
[0024] Example 2: The experimental conditions and steps are the same as in Example 1, except that 1-butyl-3-methylimidazole chloride is changed to 1-ethyl-3-methylimidazole bromide to obtain 11.93 g of terephthalic acid product . The degradation rate of PET is 99%, and the yield of terephthalic acid is 92.0%.
Embodiment 3
[0025] Example 3: The experimental conditions and steps are the same as in Example 1, except that 1-butyl-3-methylimidazole chloride is changed to 1-ethyl-3-picoline bromide to obtain 11.87 g of terephthalic acid product . The degradation rate of PET is 98.2%, and the yield of terephthalic acid is 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com