Acenaphtho-heterocycles compounds and application thereof in preparation of BH3 analogue Bcl-2 family protein inhibitor
A technology of heterocycles and compounds, applied in the field of simulating BH3-only proteins, can solve problems such as insufficient BH3 similarity, toxic side effects, and limited use range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
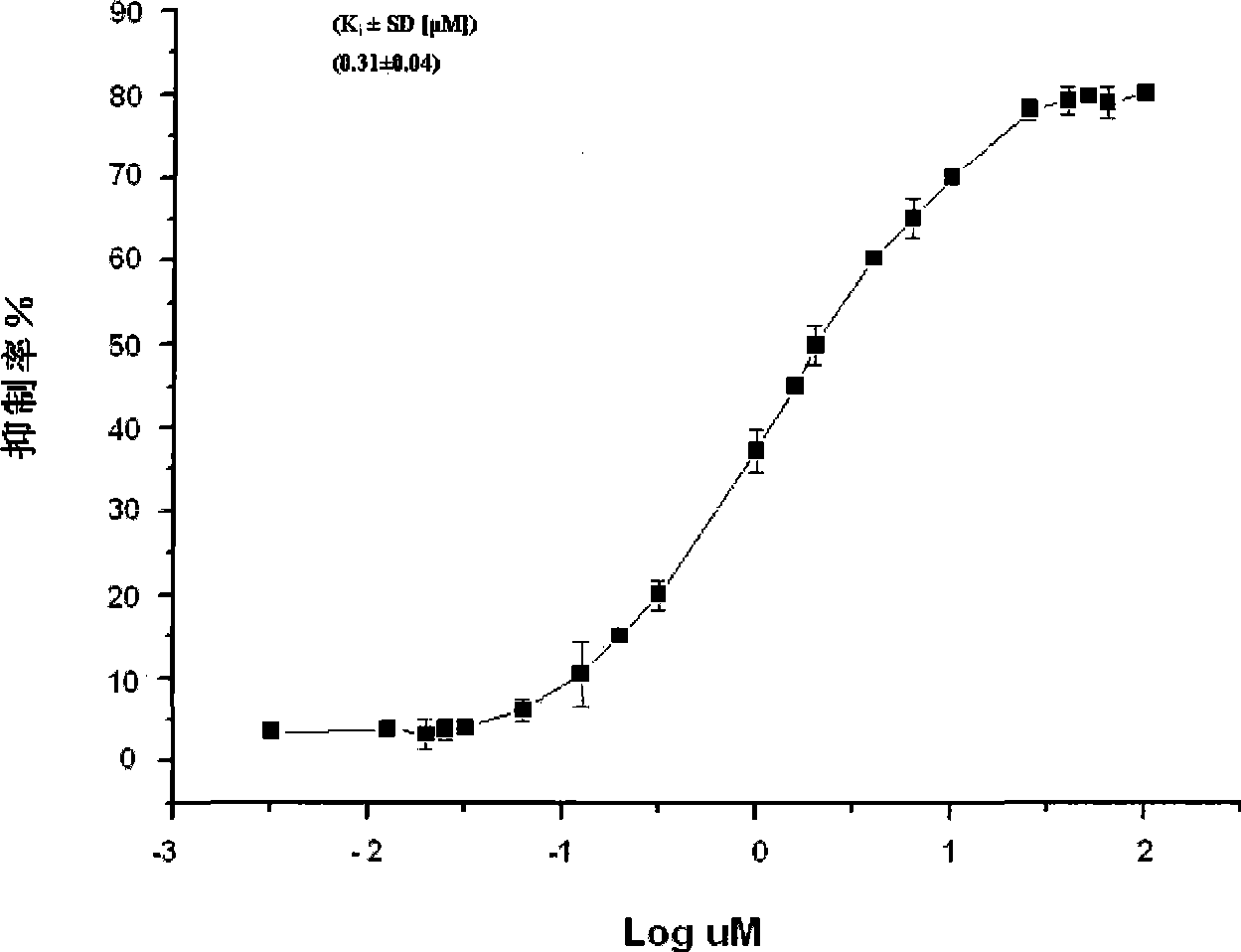
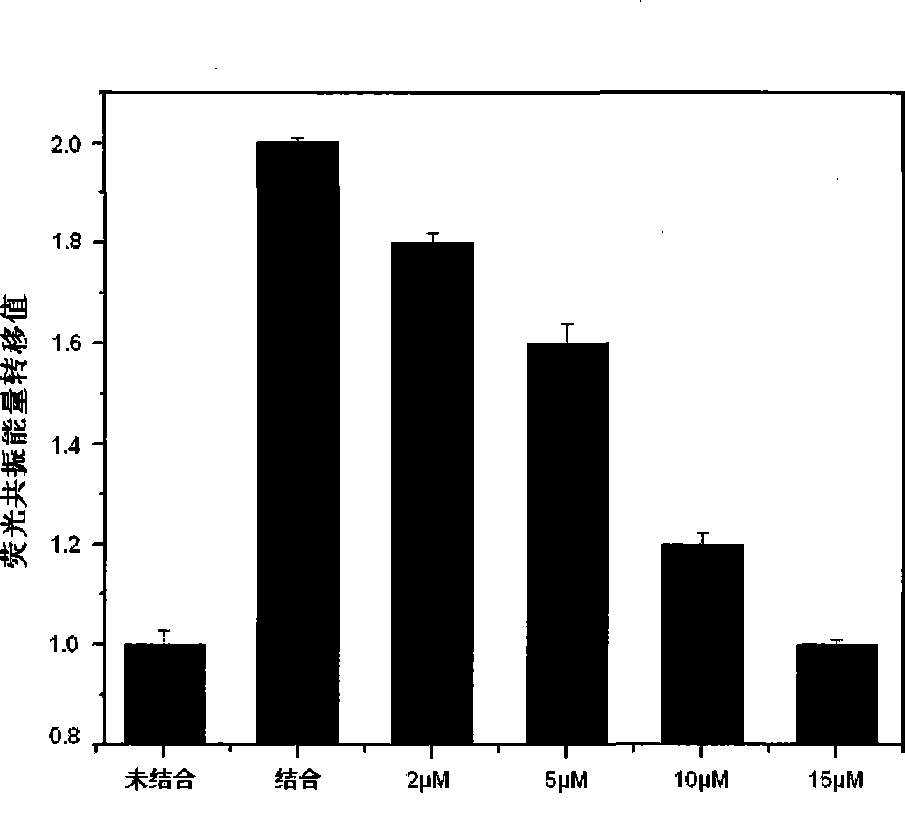
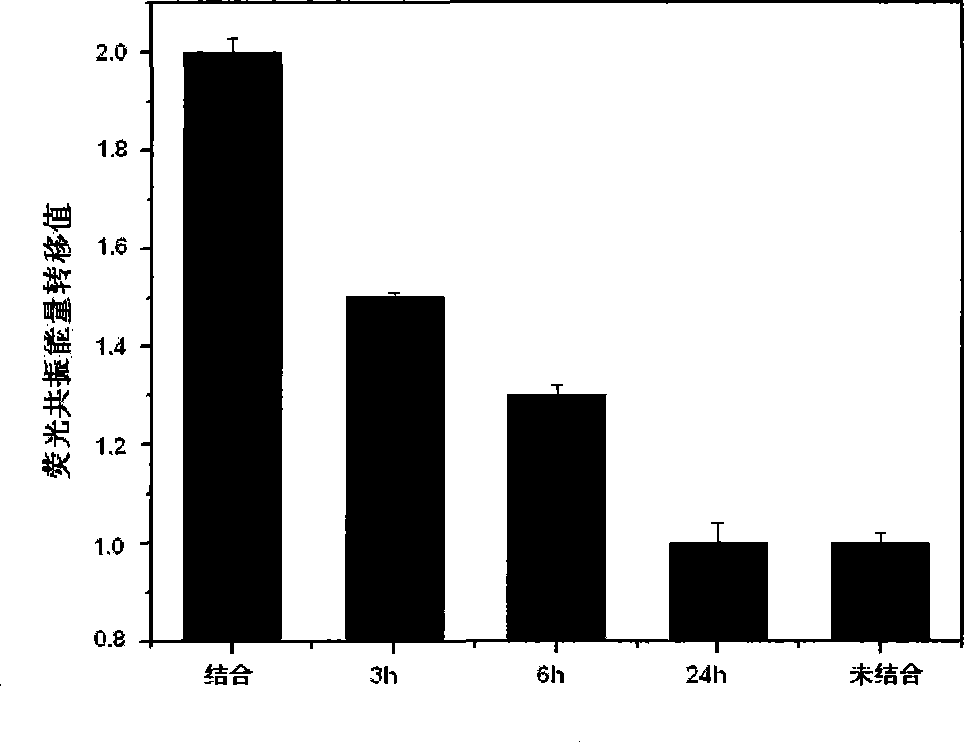
Image
Examples
Embodiment 1
[0039]
[0040]Add 1 gram of 8-oxo-8H acenaphtho[1,2-b]pyrrole-9-carbonitrile and 0.47 gram of p-cresol to 50 milliliters of acetonitrile, stir at reflux for 3 hours, evaporate part of the solvent, and separate the product by chromatography 3-(p-Tolyloxy)-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile, yield 40%. M.p.232-233°C; 1H NMR (400M, CDCl3): δ8.916(dd, J=8.8Hz, 1H), 8.623(d, J=8.8Hz, 1H), 8.447(d, J=6.4Hz, 1H ), 7.859(t, J=8.0Hz, 1H), 8.324(d, J=8.4Hz, 2H), 7.101(d, J=8.4Hz, 2H), 7.016(d, J=8.4Hz, 2H), 3.256(s, 3H).
Embodiment 2
[0042] Weigh 0.93g of phenoxyacenaphthenequinone, dissolve 0.3g of malononitrile in dichloromethane, add to a silica gel column, rinse quickly, spin dry after passing through the column to obtain a red solid. 10.1 g was weighed, and the yield was 92%. Take 0.6g 3-phenoxy-(2-oxo-2hydro-acenaphthene)-malononitrile, add 0.08g K 2 CO 3 , 20ml of acetonitrile was heated to reflux for 3h, and the reaction solution was spin-dried after the reaction was completed. Chromatographic column separation (CH 2 Cl 2 : Petroleum ether=2:1) an orange-red solid was obtained, and the isomer ratio of NMR was 1:0.3. Two isomers were separated using the preparative liquid phase.
[0043]
[0044] The first component A: M.p.265-267°C; 1 H NMR (400M, CDCl 3 ): δ 8.927(d, J=8.0Hz, 1H), 8.630(d, J=8.8Hz, 1H), 8.450(d, J=7.2Hz, 1H), 7.876(t, J=8.0Hz, 1H) , 7.754(t, J=8.0Hz, 2H), 7.392(t, J=7.6Hz, 1H), 7.233(d, J=7.6Hz, 2H), 7.028(d, J=8.4Hz, 1H).
[0045] The second component B: M.p.282-283...
Embodiment 3
[0047] Weigh 1.0 g of p-tolyloxyacenaphthenequinone, dissolve 0.3 g of malononitrile in dichloromethane, add to a silica gel column, rinse quickly, spin dry after passing through the column to obtain a red solid. Weighed 11.2 g, yield 93%. Take 0.7g 3-phenoxy-(2-oxo-2hydro-acenaphthene)-malononitrile, add 0.08g K 2 CO 3 , 20ml of acetonitrile was heated to reflux for 4h, and the reaction solution was spin-dried after the reaction was completed. Chromatographic column separation (CH 2 Cl 2 : Petroleum ether=1:1) to obtain an orange-red solid, and the ratio of isomers was 1:0.4 by NMR. Two isomers were separated using the preparative liquid phase.
[0048]
[0049] The first component A: M.p.232-233°C; 1 H NMR (400M, CDCl 3 ): δ 8.916(dd, J=8.8Hz, 1H), 8.623(d, J=8.8Hz, 1H), 8.447(d, J=6.4Hz, 1H), 7.859(t, J=8.0Hz, 1H) , 8.324(d, J=8.4Hz, 2H), 7.101(d, J=8.4Hz, 2H), 7.016(d, J=8.4Hz, 1H), 2.351(s, 3H).
[0050] The second component B: M.p.258-260°C; 1 H NMR (400M, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com