Semi-synthetic route for the preparation of paclitaxel, docetaxel and 10-deacetylbaccatin iii from 9-dihydro-13-acetylbaccatin III
A C1-C20, C1-C12 technology, applied in the field of semi-synthetic routes of other taxane compounds, can solve the problems of no side chain, no acyl group and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
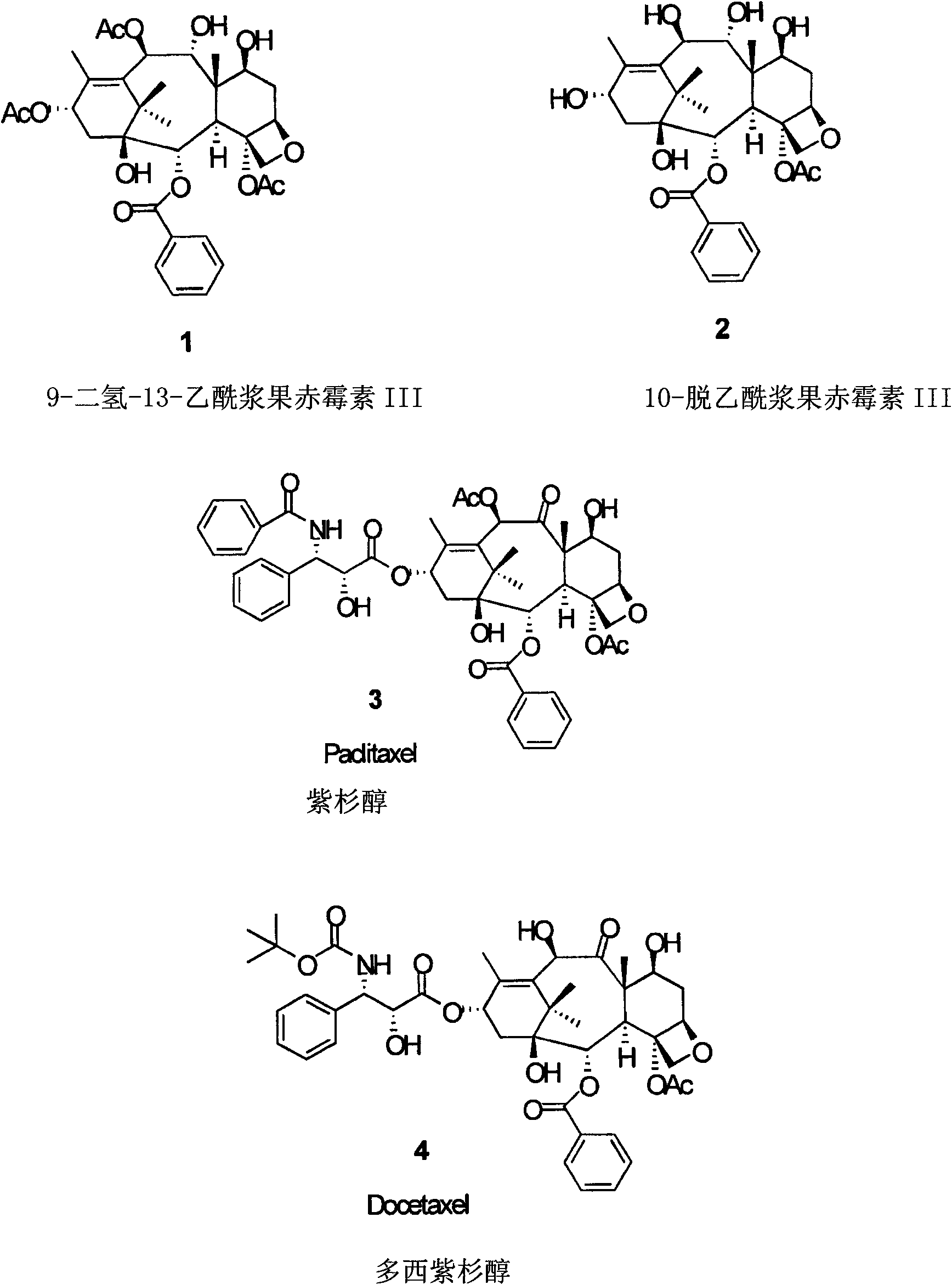
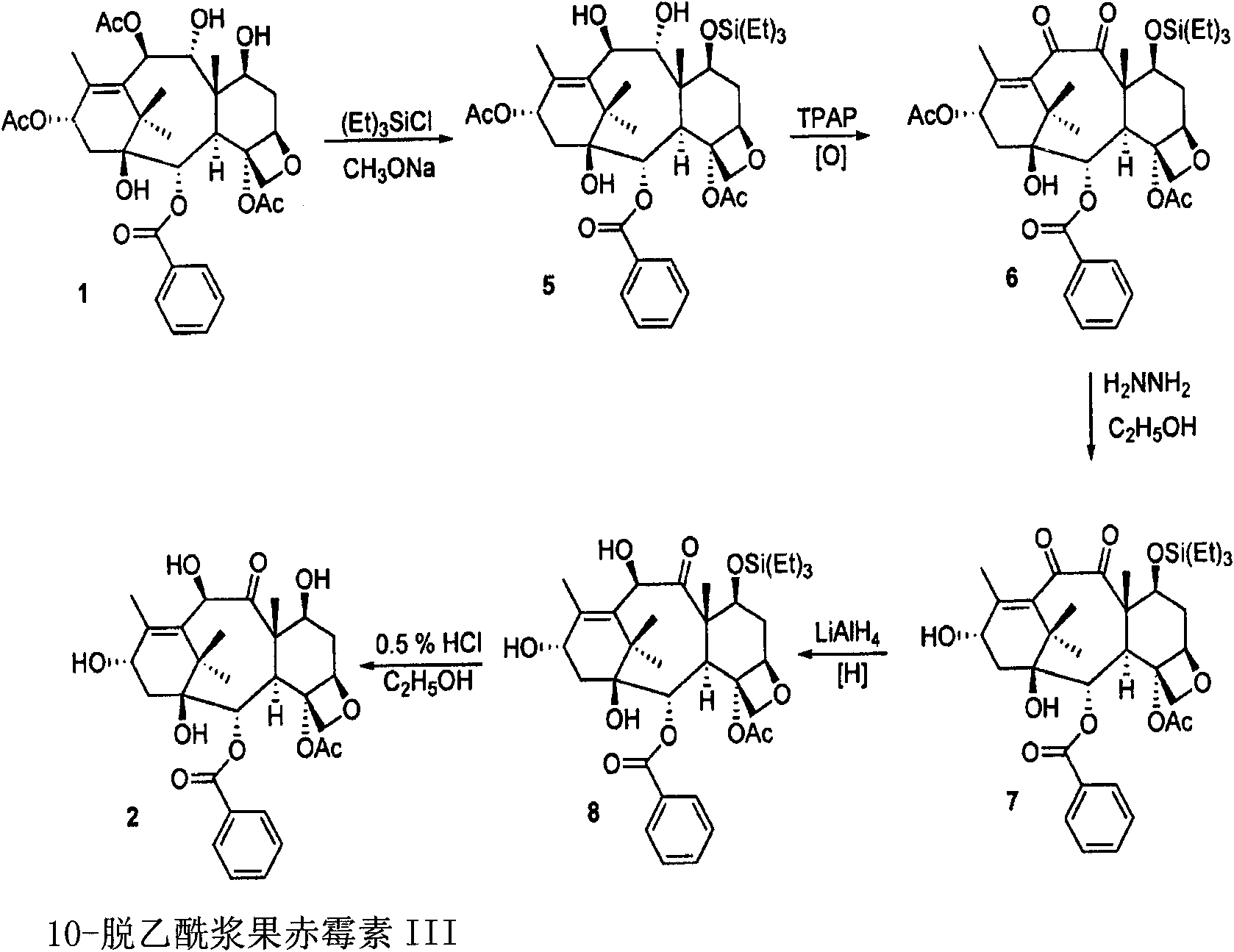
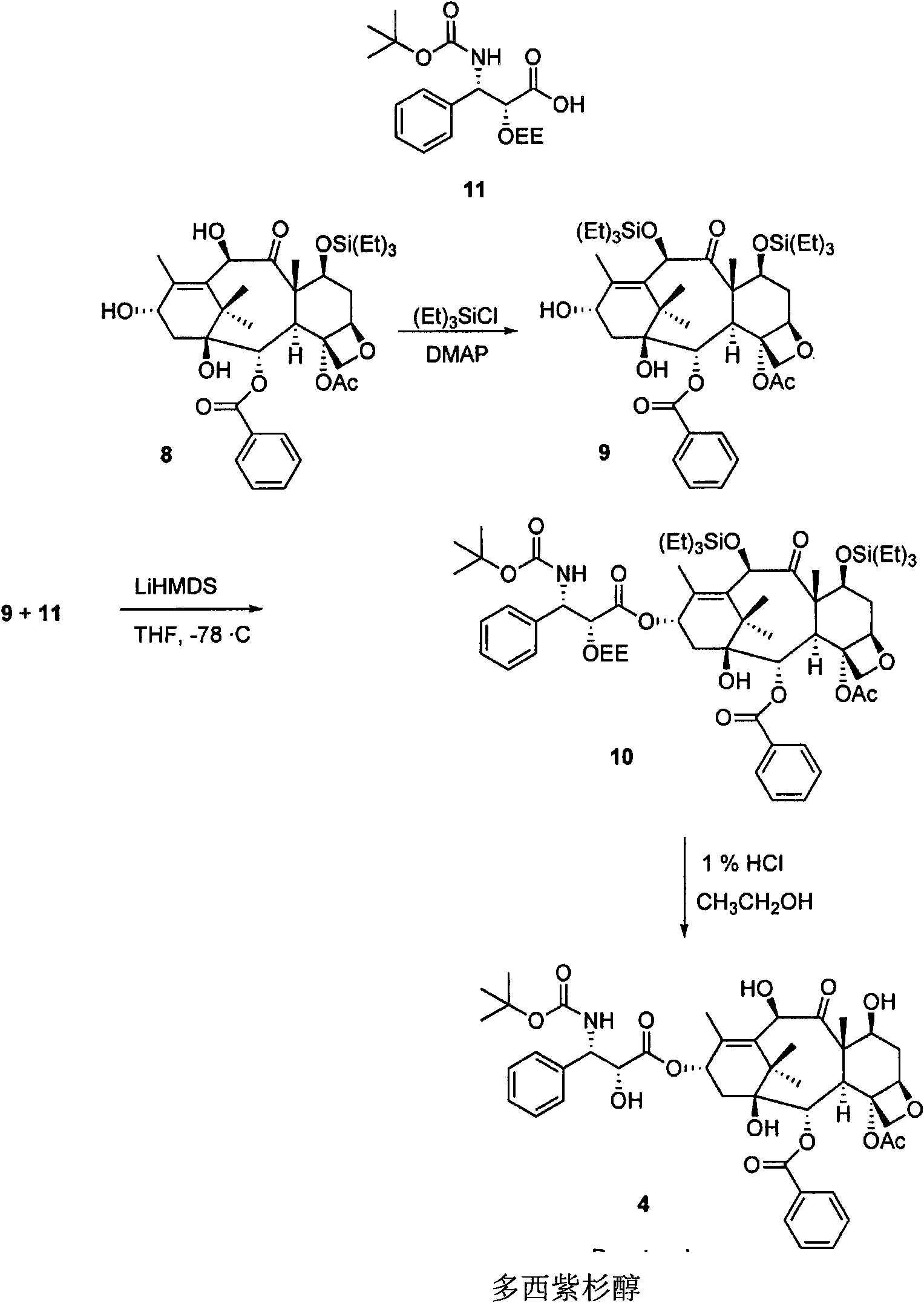
[0091] The present invention relates to taxane derivatives of 9,10-diketones, which are useful in the efficient production of paclitaxel and docetaxel analogues from 9-dihydro-13-acetylbaccatin III (9-DHAB). Formed during the chemical transformation of compounds and their intermediates. These transformations can include protection of the 7-OH of 9-DHAB, deacetylation at the C-10 position, and oxidation of the C-9 and C-10 hydroxyl groups to yield the intermediate compound 7-triethylsilyl-9 , 10-diketo-13-acetyl ccatin III ( 6 ). These processes may also include combining paclitaxel and docetaxel side chains with 9,10-diketone moieties (compound 7 ), through which paclitaxel, docetaxel and analogs thereof with different C-13 side chain structures can be synthesized, as well as intermediates thereof.
[0092] The first major aspect of the present invention is to provide a novel process for the preparation of paclitaxel and docetaxel utilizing a novel precursor, 9-dihydro-13-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com