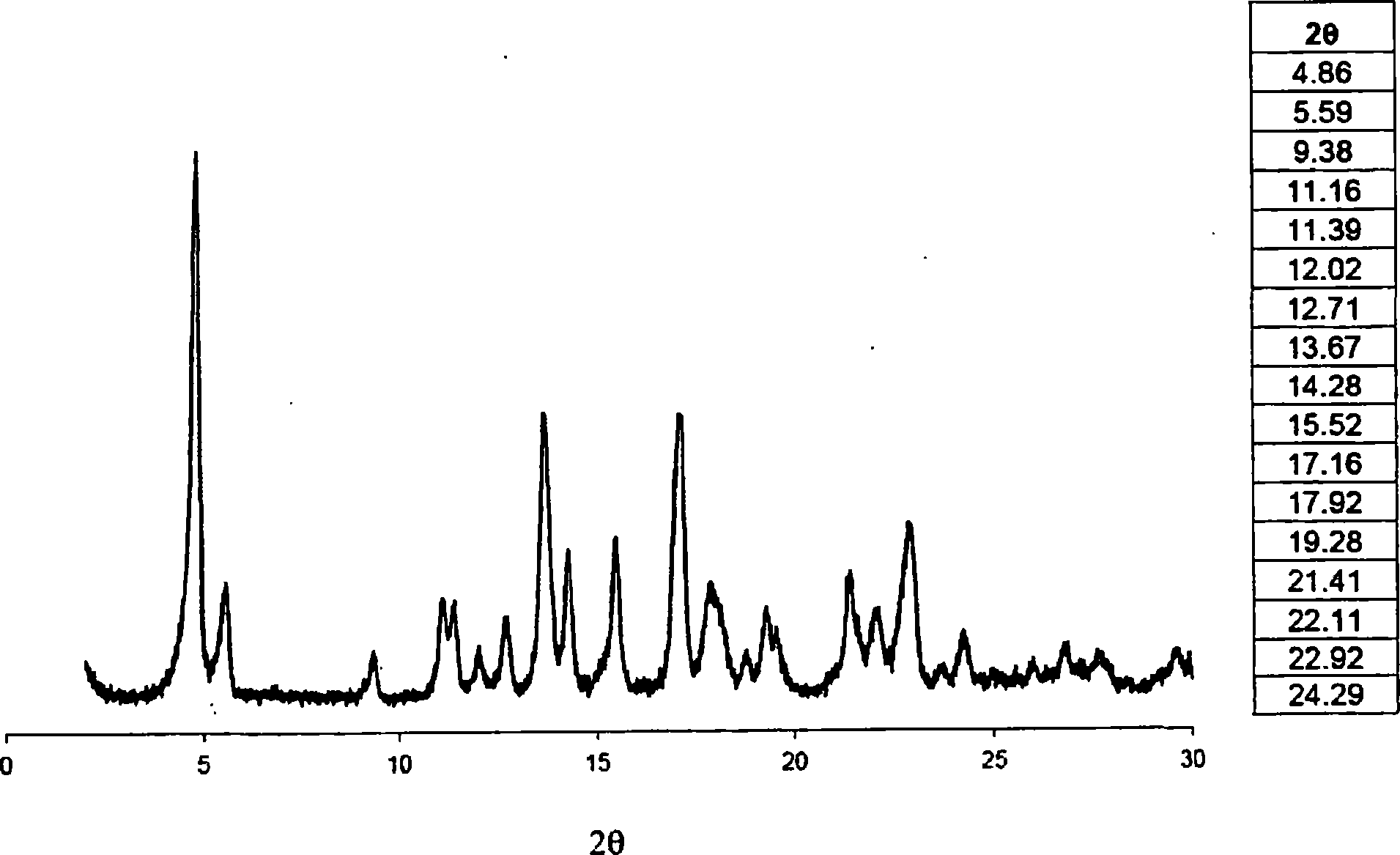
Method of producing purified rebaudioside a compositions using solvent/antisolvent crystallization
A technology for rebaudioside and composition, which is applied in the directions of food preparation, sugar derivative preparation, chemical instrument and method, etc., can solve the problem of not separating pure sweet glycosides and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
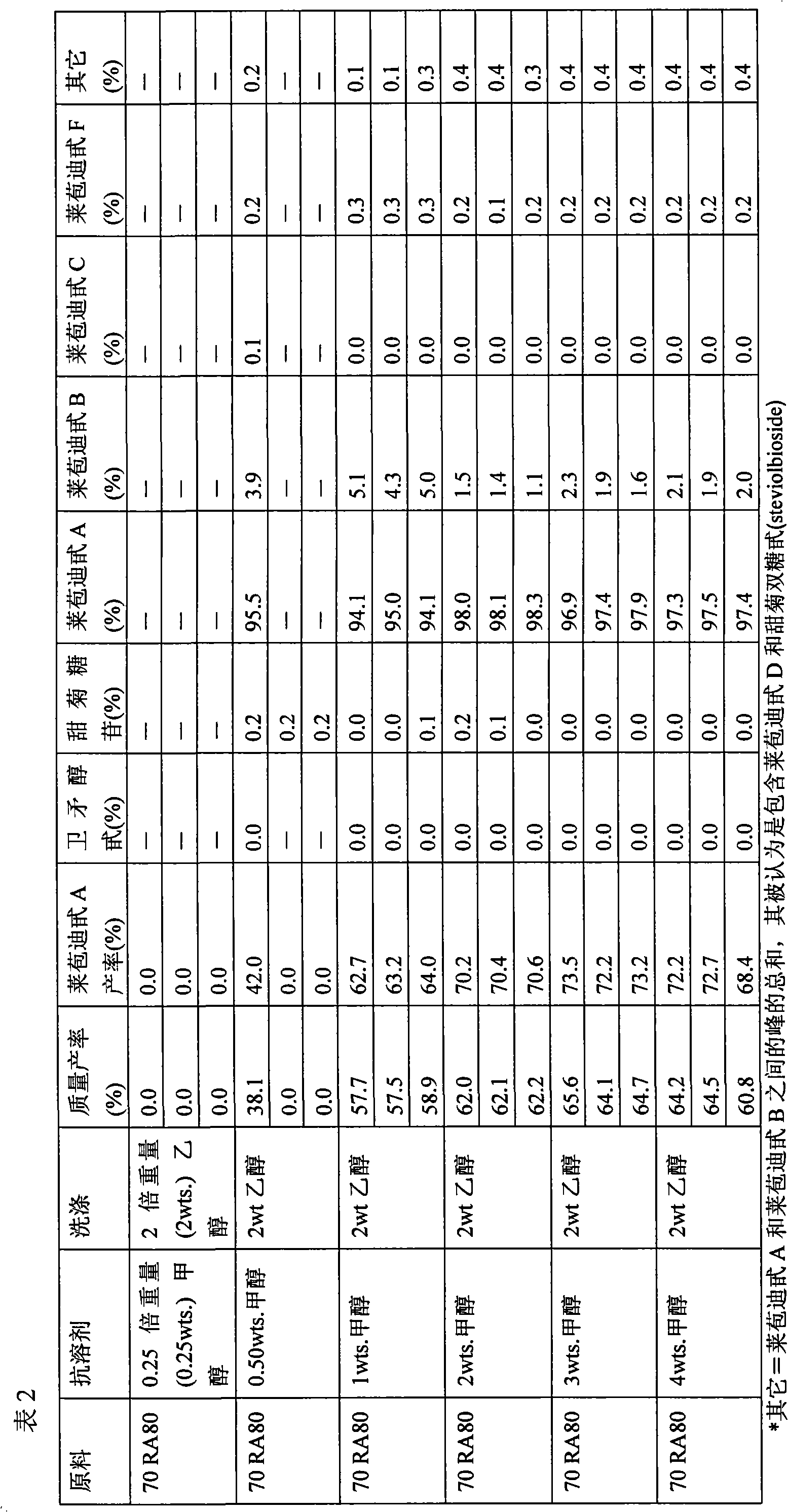
[0081] First, the initial composition 70 RA80 (see Table 1) was dissolved at 30% by weight in a solvent composition containing 50% by weight ethanol and 50% by weight water. The resulting solution was allowed to equilibrate overnight at room temperature. After equilibration, the antisolvent methanol was added to the sweetening glycoside composition in the amounts shown in Table 2 to induce crystallization. After addition of the antisolvent, crystallization was allowed to proceed overnight at room temperature. After crystallization, the solution was filtered and the resulting filter cake was washed with 2 weights of ethanol. The filter cake and supernatant samples were then dried, and the dried filter cake was analyzed by HPLC to determine the glycoside composition. The results obtained are reported in Table 2.
[0082]
Embodiment 2
[0084] First, the starting materials shown in Table 3 were dissolved at 30% by weight in a solvent composition containing 50% by weight of ethanol and 50% by weight of water. The resulting solution was allowed to equilibrate overnight at room temperature. After equilibration, 2 volumes of antisolvent were added to the sweetening glycoside composition to induce crystallization. After addition of the antisolvent, crystallization was allowed to proceed overnight at room temperature. The addition of 2 volumes of antisolvent was observed to produce crystallization in the 80% concentration of the rebaudioside A starting material, but not in sweet glycoside compositions prepared from 60% or 40% rebaudioside A starting compositions crystallization. An additional volume of antisolvent was added to the glycoside compositions prepared from the 60% and 40% rebaudioside A starting compositions. The extra volume of ethanol antisolvent caused crystallization to occur in two of the three s...
Embodiment 3
[0087]RA20 (see Table 1) was dissolved in a solution of 50% ethanol and 50% water to yield a solution containing 30% by weight RA20. The solution was diluted with 3 times the weight of methanol, filtered, and washed with 2 times the weight of methanol. The crystals accounted for 56% by weight of the original material and were rich in steviol glycosides. What remains is the filtrate and is rich in rebaudioside A. The purity of the obtained crystals and filtrate is given in Table 4.
[0088] Table 4
[0089]
[0090] * Other = sum of peaks between rebaudioside A and rebaudioside B, which is believed to include rebaudioside D and steviolbioside
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com